Abstract
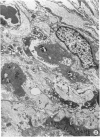
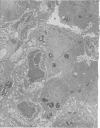
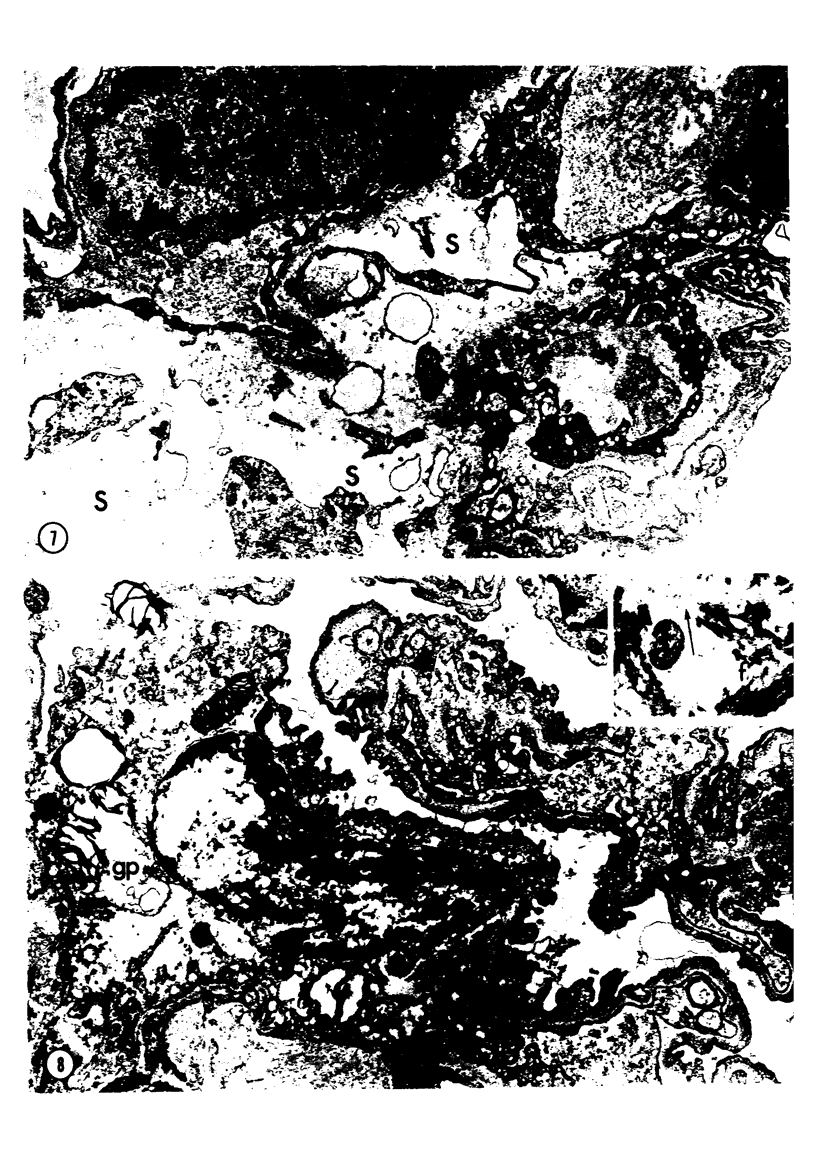
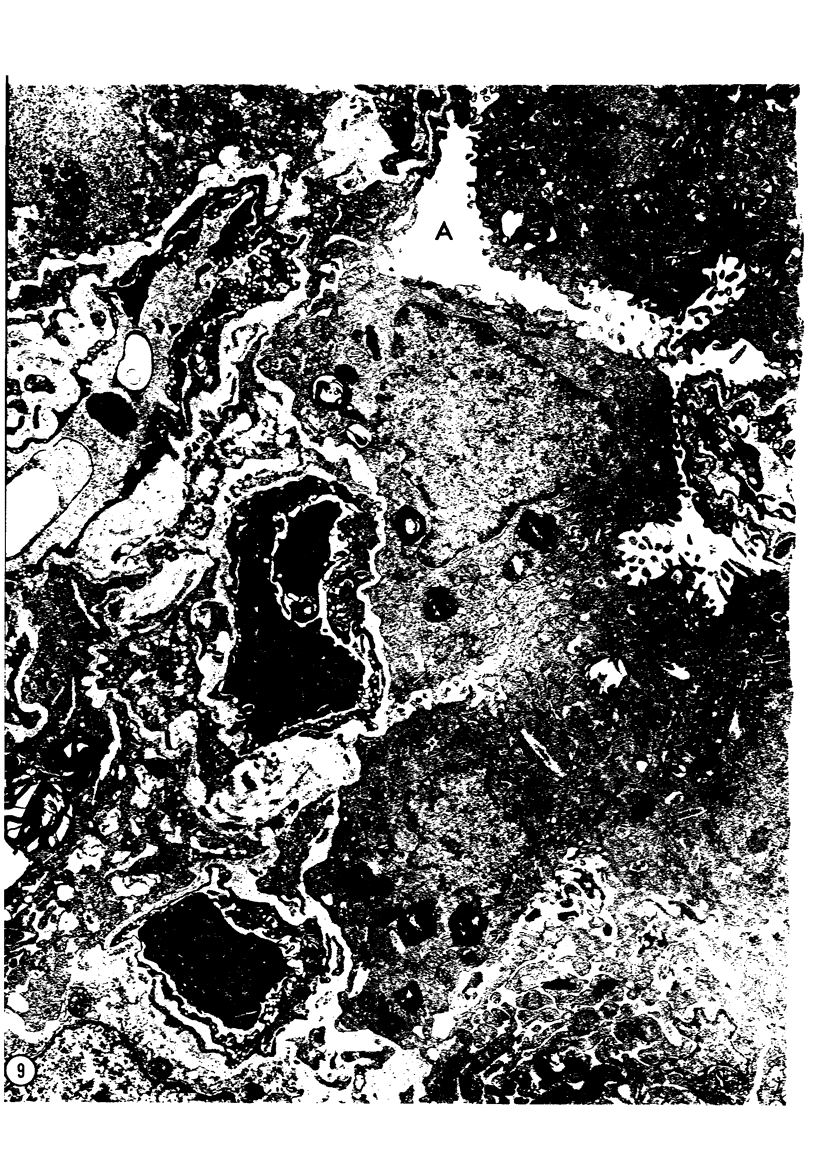
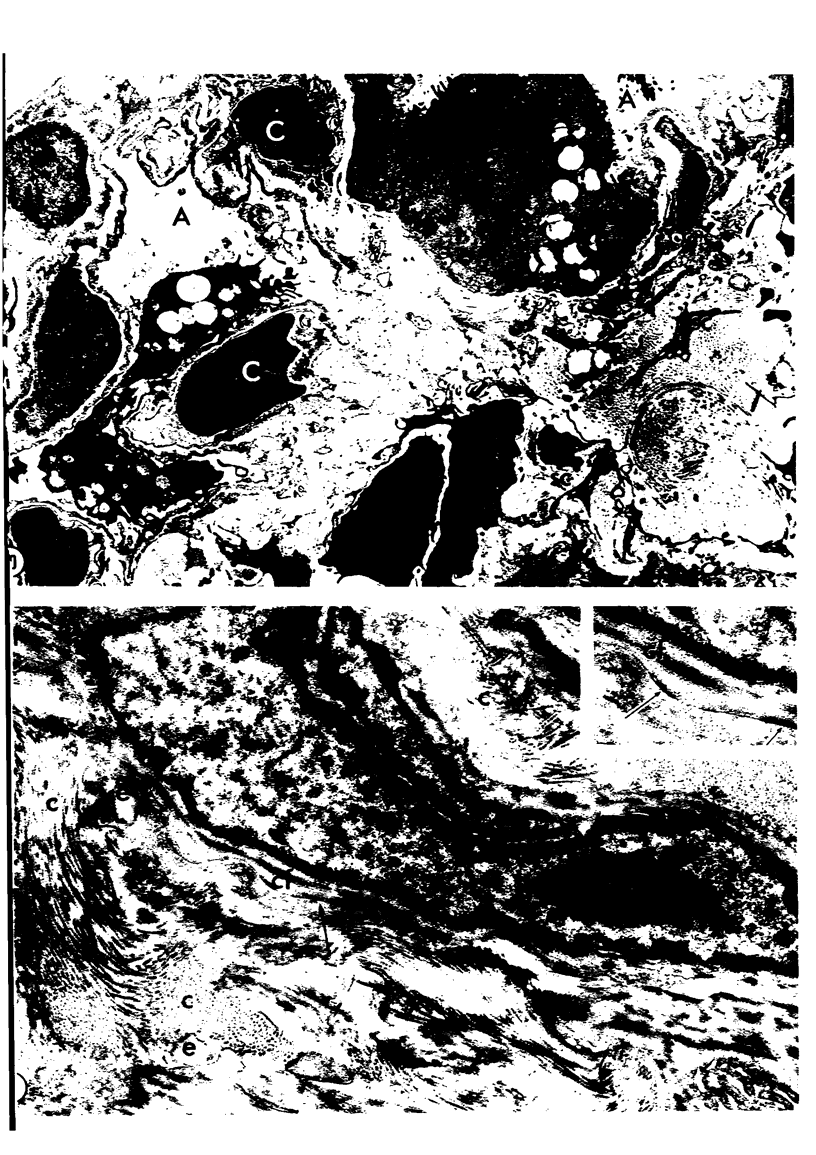
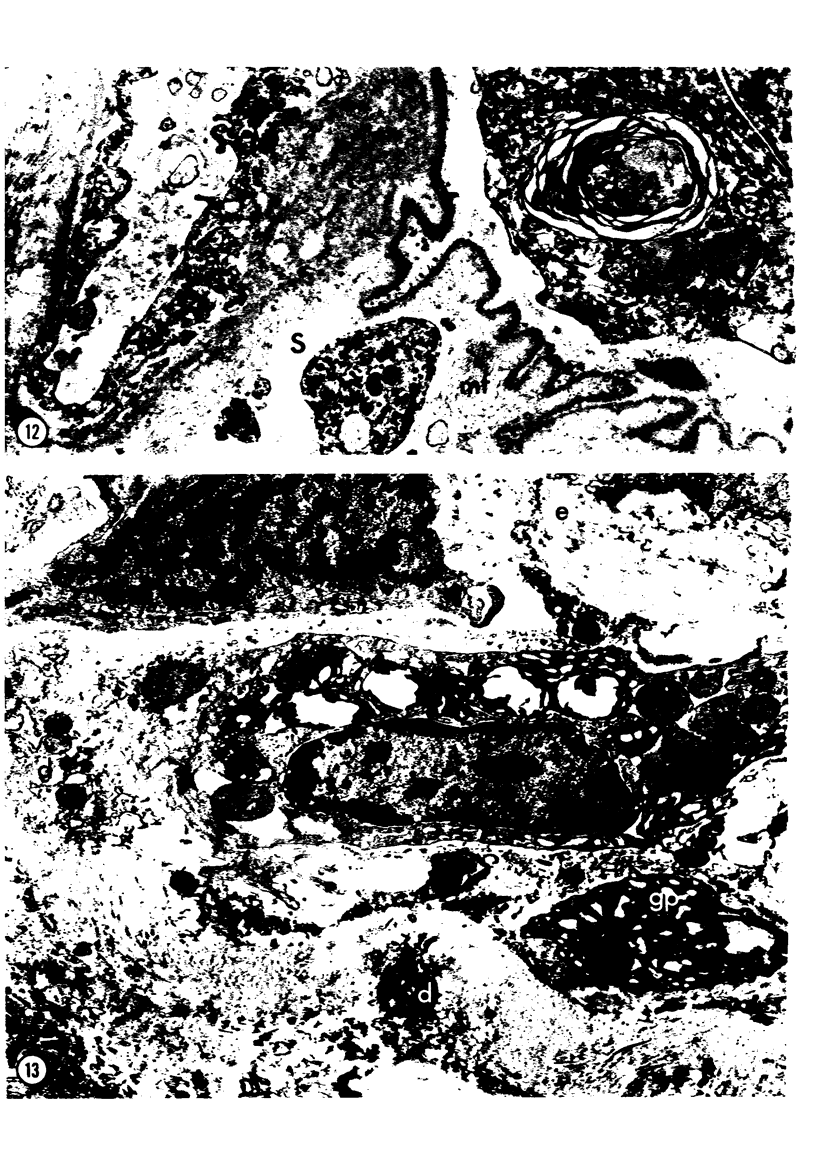
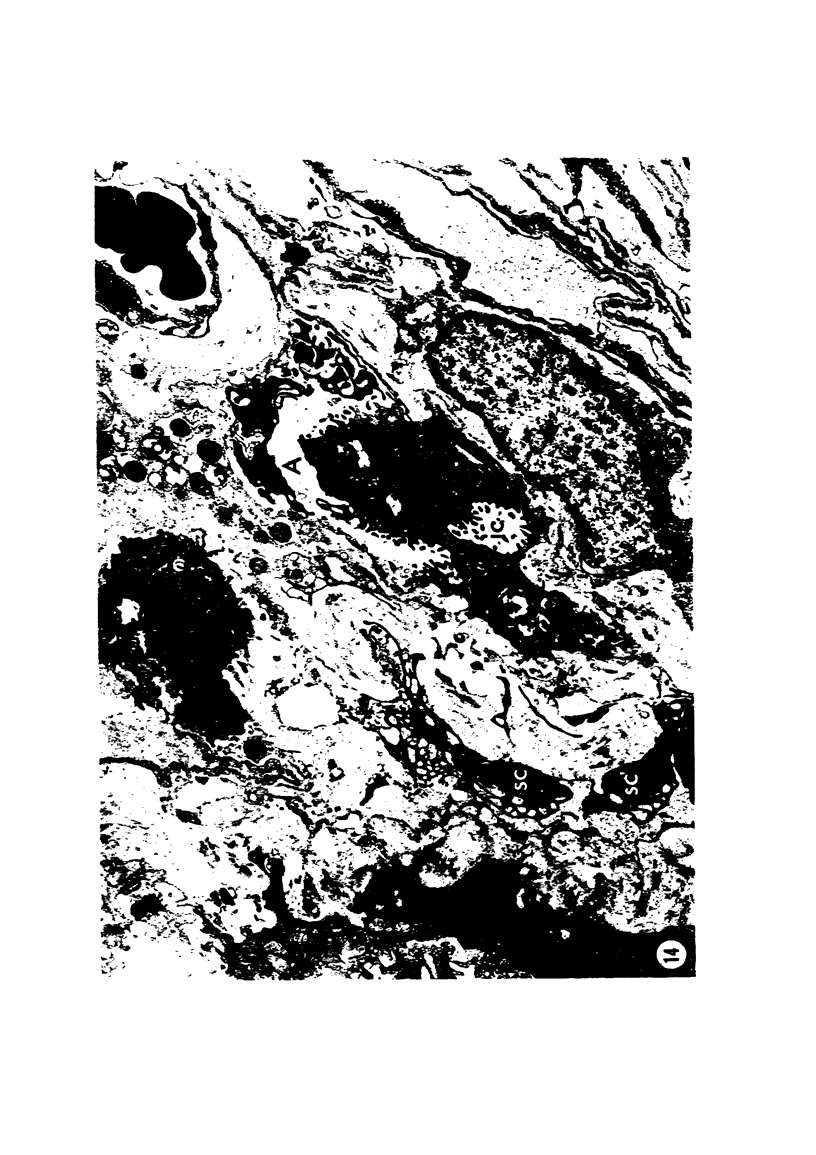
Adult rats received, intraperitoneally, 20 mg/100 g body weight of cyclophosphamide and were killed 1, 2, 3, 4, 7, 14, 21, and 28 days thereafter. Lung samples were studied by light and electron microscopy. Light microscopy revealed septal and intraalveolar hemorrhages at 2 days and hyaline membranes at 4 days. At 1 to 2 weeks the alveoli were reepithelialized; beyond these intervals there was septal thickening with increased septal cells and interstitial substance. Electron microscopy showed capillary endothelial blebs, membranous pneumocyte injury and sloughing, and severe septal edema at 1 to 2 days. At 4 days some granular pneumocytes appeared altered. At 1 week the alveoli were reepithelialized by prominent granular pneumocytes. Beyond these intervals there was septal thickening with abundant septal cells, debris, collagen, elastin and microfibrils. Some septal elements showed features consistent with "contractile interstitial cells." There was also alveolar collapse indicated by "trapped" granular pneumocytes surrounded by septal cells and fibers. Occasional granular pneumocytes showed large intracytoplasmic cavities. Cyclophosphamide can induce severe injury involving all alveolar components. The partly denuded alveoli are reepithelialized by proliferating granular pneumocytes, thus confirming their importance in alveolar repair. The subsequent development of sclerosing alveolitis suggests that cyclophosphamide may offer a useful experimental model for the study of alveolar injury and repair. The role of the septal "contractile interstitial cells" in the development of septal fibrosis and the possibility that these lesions are reversible remain to be clarified.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974 Nov;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H., Wyatt J. P. Oxygen poisoning in mice. Ultrastructural and surfactant studies during exposure and recovery. Arch Pathol. 1970 Nov;90(5):463–472. [PubMed] [Google Scholar]
- André R., Rochant H., Dreyfus B., Duhamel G., Péchère J. C. Fibrose interstitielle diffuse du poumon au cours d'une maladie de Hodgkin traitée par des doses élevées d'endoxan. Bull Mem Soc Med Hop Paris. 1967 Nov 3;118(12):1133–1141. [PubMed] [Google Scholar]
- Ansell G. Radiological manifestations of drug-induced disease. Clin Radiol. 1969 Apr;20(2):133–148. doi: 10.1016/s0009-9260(69)80162-5. [DOI] [PubMed] [Google Scholar]
- Balis J. U., Delivoria M., Conen P. E. Maturation of postnatal human lung and the idiopathic respiratory distress syndrome. Lab Invest. 1966 Mar;15(3):530–546. [PubMed] [Google Scholar]
- Beck R. E., Boyes D. A. Treatment of 126 cases of advanced ovarian carcinoma with cyclophosphamide. Can Med Assoc J. 1968 Mar 16;98(11):539–541. [PMC free article] [PubMed] [Google Scholar]
- Bedrossian C. W., Luna M. A., Mackay B., Lichtiger B. Ultrastructure of pulmonary bleomycin toxicity. Cancer. 1973 Jul;32(1):44–51. doi: 10.1002/1097-0142(197307)32:1<44::aid-cncr2820320106>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Bonikos D. S., Koss L. G. Acute effects of cyclophosphamide on rat urinary bladder muscle. Arch Pathol. 1974 Apr;97(4):242–245. [PubMed] [Google Scholar]
- Brettner A., Heitzman E. R., Woodin W. G. Pulmonary complications of drug therapy. Radiology. 1970 Jul;96(1):31–38. doi: 10.1148/96.1.31. [DOI] [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- Davies P. D. Drug-induced lung disease. Br J Dis Chest. 1969 Apr;63(2):59–70. doi: 10.1016/s0007-0971(69)80031-8. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Fishman A. P., Pietra G. G. Handling of bioactive materials by the lung (first of two parts). N Engl J Med. 1974 Oct 24;291(17):884–889. doi: 10.1056/NEJM197410242911706. [DOI] [PubMed] [Google Scholar]
- Fishman A. P., Pietra G. G. Handling of bioactive materials by the lung (second of two parts). N Engl J Med. 1974 Oct 31;291(18):953–959. doi: 10.1056/NEJM197410312911808. [DOI] [PubMed] [Google Scholar]
- Fleischman R. W., Baker J. R., Thompson G. R., Schaeppi U. H., Illievski V. R., Cooney D. A., Davis R. D. Bleomycin-induced interstitial pneumonia in dogs. Thorax. 1971 Nov;26(6):675–682. doi: 10.1136/thx.26.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972 Jan;66(1):131–146. [PMC free article] [PubMed] [Google Scholar]
- Goldenberg V. E., Buckingham S., Sommers S. C. Pulmonary alveolar lesions in vagotomized rats. Lab Invest. 1967 May;16(5):693–705. [PubMed] [Google Scholar]
- Goldenberg V. E., Warren S., Chute R., Besen M. Radiation pneumonitis in single and parabiotic rats. I. Short term effects of supralethal total body irradiation. Lab Invest. 1968 Mar;18(3):215–226. [PubMed] [Google Scholar]
- Gould V. E., Gleason T. H., Winterscheid L. C. Desquamative interstitial pneumonia. Chest. 1971 Mar;59(3):349–352. doi: 10.1378/chest.59.3.349. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Smuckler E. A. Alveolar injury in acute carbon tetrachloride intoxication. Arch Intern Med. 1971 Jul;128(1):109–117. [PubMed] [Google Scholar]
- Gould V. E., Tosco R., Wheelis R. F., Gould N. S., Kapanci Y. Oxygen pneumonitis in man. Ultrastructural observations on the development of alveolar lesions. Lab Invest. 1972 May;26(5):499–508. [PubMed] [Google Scholar]
- HEINEMANN H. O. Respiration and circulation in patients with portal cirrhosis of the liver. Circulation. 1960 Jul;22:154–159. doi: 10.1161/01.cir.22.1.154. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Slesers A., Aschenbrenner I. Pulmonary intra-alveolar histiocytosis induced by drugs. Toxicol Appl Pharmacol. 1973 Sep;26(1):72–85. doi: 10.1016/0041-008x(73)90087-2. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Chauvet M. La pneumonie desquamative interstitielle. Schweiz Med Wochenschr. 1967 Sep 16;97(37):1199–1208. [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kaplan H. P., Robinson F. R., Kapanci Y., Weibel E. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. 1969 Jan;20(1):94–100. [PubMed] [Google Scholar]
- Kirschner R. H., Esterly J. R. Pulmonary lesions associated with busulfan therapy of chronic myelogenous leukemia. Cancer. 1971 May;27(5):1074–1080. doi: 10.1002/1097-0142(197105)27:5<1074::aid-cncr2820270511>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss L. G., Melamed M. R., Mayer K. The effect of busulfan on human epithelia. Am J Clin Pathol. 1965 Oct;44(4):385–397. doi: 10.1093/ajcp/44.4.385. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin P., Koss L. G. Effects of a single dose of cyclophosphamide on various organs in the rat. 3. Electron microscopic study of the liver. Am J Pathol. 1971 Feb;62(2):159–168. [PMC free article] [PubMed] [Google Scholar]
- Lavin P., Koss L. G. Effects of a single dose of cyclophosphamide on various organs in the rat. IV. Electron microscopic study of the renal tubules. Am J Pathol. 1971 Feb;62(2):169–180. [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971 Aug 6;173(3996):548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- OLINER H., SCHWARTZ R., RUBIO F., DAMESHEK W. Interstitial pulmonary fibrosis following busulfan therapy. Am J Med. 1961 Jul;31:134–139. doi: 10.1016/0002-9343(61)90229-7. [DOI] [PubMed] [Google Scholar]
- Rodin A. E., Haggard M. E., Travis L. B. Lung changes and chemotherapeutic agents in childhood. Report of a case associated with cyclophosphamide therapy. Am J Dis Child. 1970 Oct;120(4):337–340. doi: 10.1001/archpedi.1970.02100090111012. [DOI] [PubMed] [Google Scholar]
- Rosenow E. C., 3rd The spectrum of drug-induced pulmonary disease. Ann Intern Med. 1972 Dec;77(6):977–991. doi: 10.7326/0003-4819-77-6-977. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irle C., Statkov P. R., Majno G. Myofibroblasts in an avascular fibrous tissue. Lab Invest. 1973 Aug;29(2):197–206. [PubMed] [Google Scholar]
- Shimosato Y., Baba K., Watanabe S. [Pulmonary lesions produced by antitumor drugs--studies on autopsy cases]. Gan No Rinsho. 1971;17(1):21–34. [PubMed] [Google Scholar]
- Smith H. C., Gould V. F., Cheney F. W., Butler J. Pathogenesis of hemodynamic pulmonary edema in excised dog lungs. J Appl Physiol. 1974 Dec;37(6):904–911. doi: 10.1152/jappl.1974.37.6.904. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Vracko R. Significance of basal lamina for regeneration of injured lung. Virchows Arch A Pathol Pathol Anat. 1972;355(3):264–274. doi: 10.1007/BF00551062. [DOI] [PubMed] [Google Scholar]
- WESTON J. T., GUIN G. H. Epithelial atypias with chemotherapy in 100 acute childhood leukemias. Cancer. 1955 Jan-Feb;8(1):179–186. doi: 10.1002/1097-0142(1955)8:1<179::aid-cncr2820080125>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]