Abstract
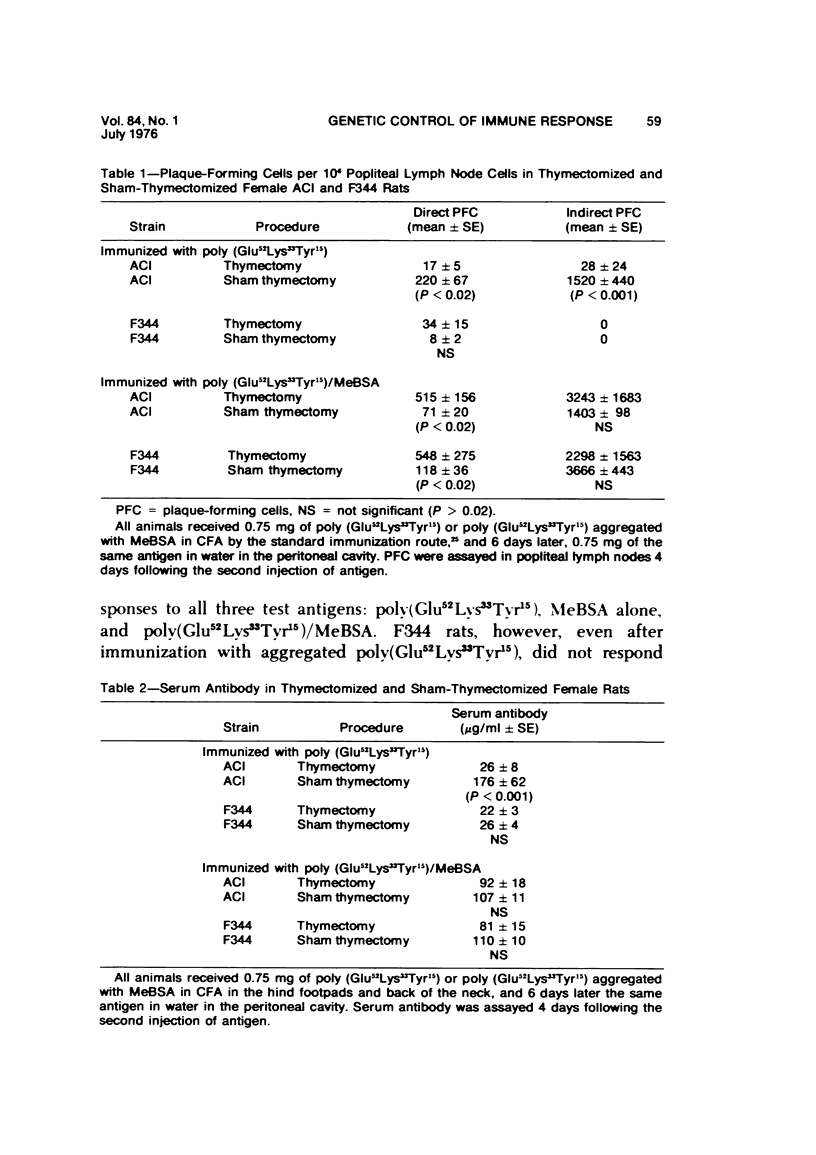
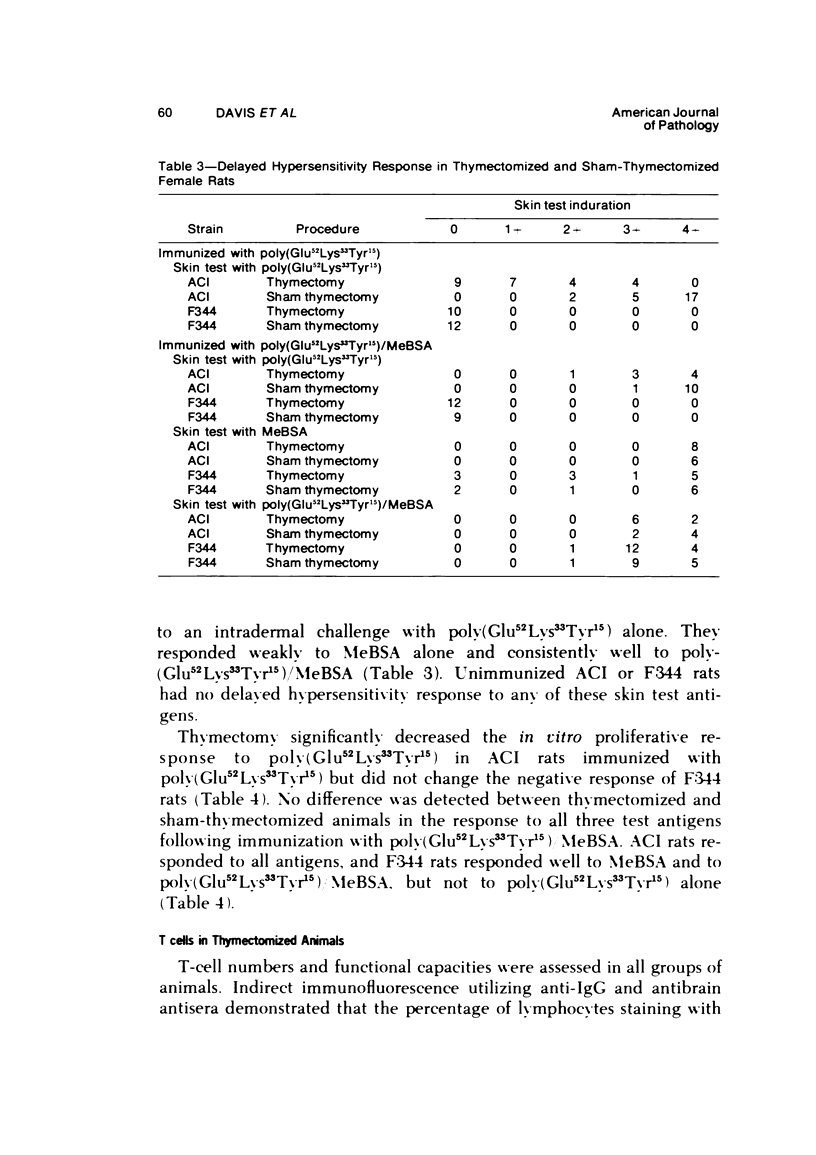
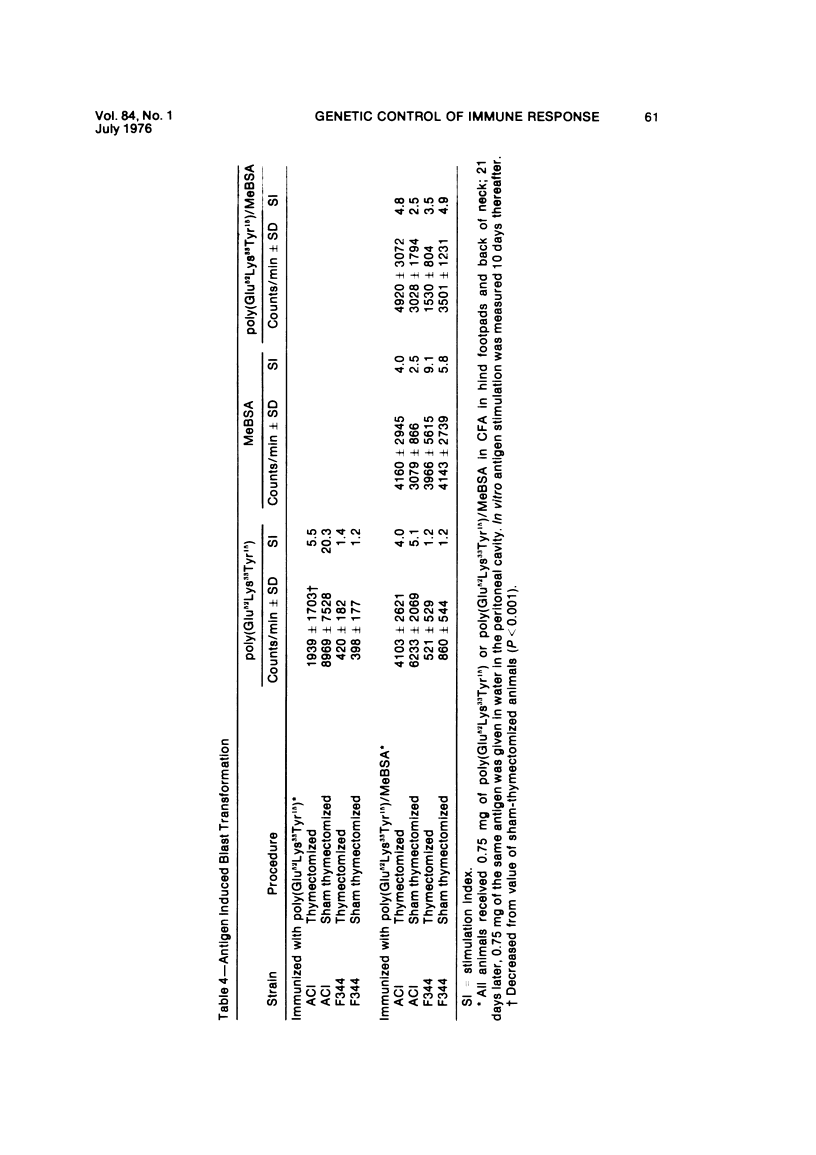
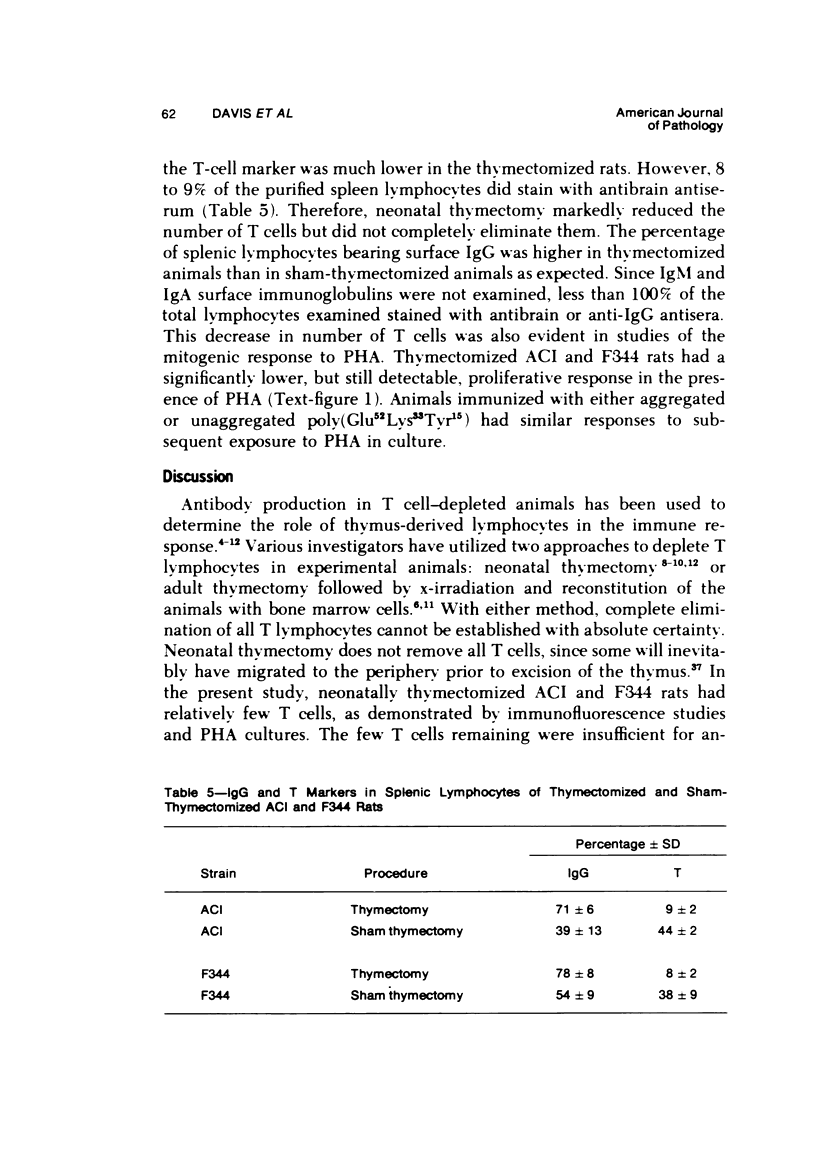
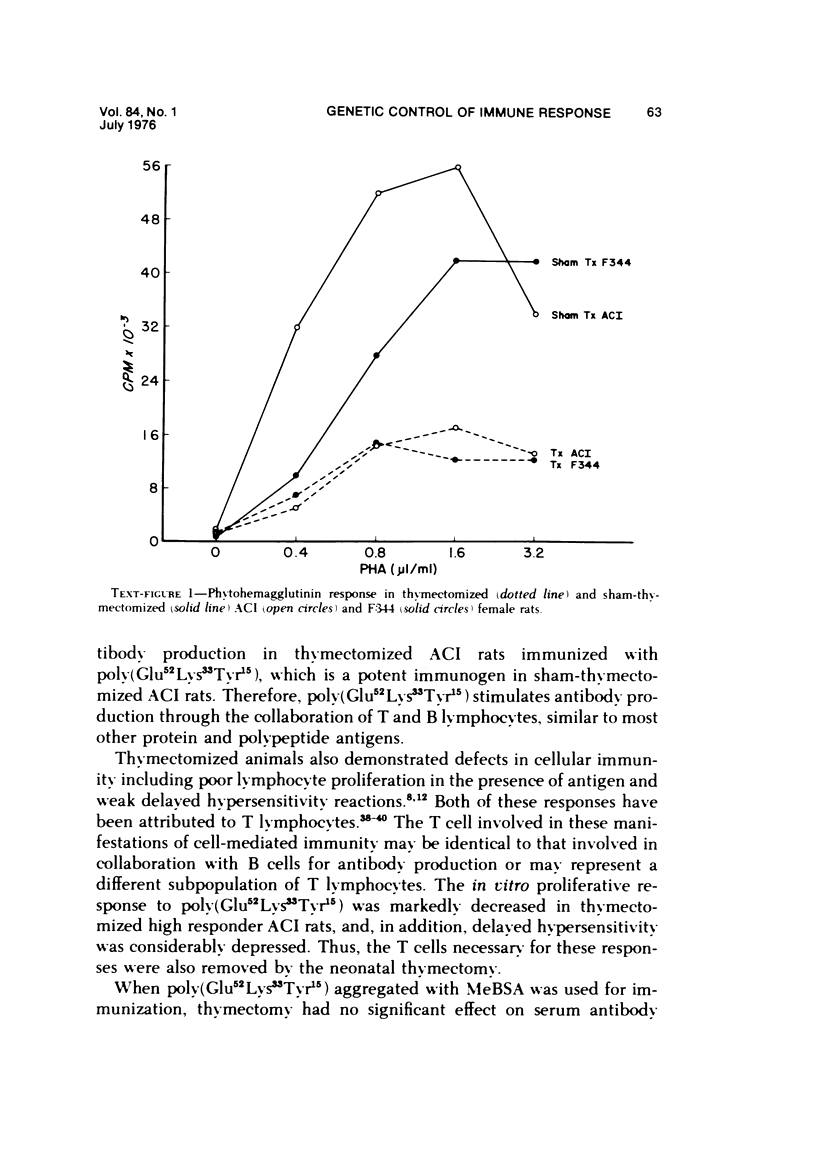
The immune response to poly (Glu52Lys33Tyr15) is under polygenic control and linked to the major histocompatibility complex of the rat. Aggregation of this antigen with methylated bovine serum albumin (MeBSA) eliminates the expression of genetic control by increasing the response of low responders and decreasing that of high responders. Humoral and cellular aspects of the immune response to both unaggregated and aggregated poly (Glu52Lys33Tyr15) were investigated in neonatally thymectomized high-responder ACI and low-responder F344 rats. T cells are necessary for responses to unaggregated poly (Glu52Lys33Tyr15) since thymectomy significantly decreased numbers of antibody-forming cells and serum antibody levels, and delayed hypersensitivity responses and antigen-induced in vitro proliferation. However, thymectomy had no significant effect on these parameters of immune responsiveness in either ACI or F344 rats immunized with poly (Glu52Lys33Tyr15)/MeBSA. Aggregation also increased IgG production and delayed hypersensitivity and antibody affinity in low responders.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNASON B. G., JANKOVIC B. D., WAKSMAN B. H., WENNERSTEN C. Role of the thymus in immune reactions in rats. II. Suppressive effect of thymectomy at birth on reactions of delayed (cellular) hypersensitivity and the circulating small lymphocyte. J Exp Med. 1962 Aug 1;116:177–186. doi: 10.1084/jem.116.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Braun D. G., Kindred B., Jacobson E. B. Streptococcal group A carbohydrate antibodies in mice: evidence for strain differences in magnitude and restriction of the response, and for thymus dependence. Eur J Immunol. 1972 Apr;2(2):138–143. doi: 10.1002/eji.1830020209. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Role of T lymphocytes in the humoral immune response. I. Proliferation of B lymphocytes in thymus-deprived mice. J Immunol. 1974 Nov;113(5):1438–1445. [PubMed] [Google Scholar]
- Diener E., O'Callaghan F., Kraft N. Immune response in vitro to Salmonella H-antigens, not affected by anti-theta serum. J Immunol. 1971 Dec;107(6):1775–1777. [PubMed] [Google Scholar]
- Douglas T. C. Occurrence of a theta-like antigen in rats. J Exp Med. 1972 Nov 1;136(5):1054–1062. doi: 10.1084/jem.136.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Silvers W. K. Genetic determinants of immunological responsiveness. Adv Immunol. 1974;18:1–66. doi: 10.1016/s0065-2776(08)60307-7. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Bernard C. F. Quantitative micro-method for measuring antibody utilizing the bromoacetyl cellulose immunoadsorbant. Immunochemistry. 1969 Jul;6(4):567–571. doi: 10.1016/0019-2791(69)90196-7. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W. Genetic and cellular factors in the immune response. II. Evidence for the polygenic control of the antibody response from further breeding studies and from pedigree analyses. J Immunol. 1971 Apr;106(4):980–992. [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W., Stechschulte D. J., Austen K. F. Genetic and cellular factors in the immune response. I. Genetic control of the antibody response to poly Glu52 Lys33 Tyr15 in the inbred rat strains ACI and F344. J Immunol. 1970 Jul;105(1):14–28. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Grumet F. C. Genetic control of the immune response. A selective defect in immunologic (IgG) memory in nonresponder mice. J Exp Med. 1972 Jan;135(1):110–125. doi: 10.1084/jem.135.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hege J. S., Cole L. J. Antibody plaque-forming cells in unsensitized mice. Specificity and response to neonatal thymectomy, x-irradiation and PHA. J Immunol. 1967 Jul;99(1):61–70. [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M. Studies on immunological paralysis. IV. The relative contributions of continuous antibody neutralization and central inhibition to paralysis with type 3 pneumococcal polysaccharide. Proc R Soc Lond B Biol Sci. 1971 Sep 28;178(1053):417–438. doi: 10.1098/rspb.1971.0073. [DOI] [PubMed] [Google Scholar]
- JANKOVIC B. D., WAKSMAN B. H., ARNASON B. G. Role of the thymus in immune ractions in rats. I. The immunologic response to bovine serum albumin (antibody formation, Arthus reactivity, and delayed hypersensitivity) in rats thymectomized or splenectomized at various times after birth. J Exp Med. 1962 Aug 1;116:159–176. doi: 10.1084/jem.116.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Wilson D. B. Origin of immunoreactive lymphocytes in rats. Cell Immunol. 1970 Oct;1(4):430–444. doi: 10.1016/0008-8749(70)90019-5. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Benacerraf B. Genetic control of specific immune responses. Adv Immunol. 1969;11:31–74. doi: 10.1016/s0065-2776(08)60477-0. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O. Genetic control of the antibody response. 3. Qualitative and quantitative characterization of the antibody response to (T,G)-A--L in CBA and C57 mice. J Immunol. 1968 Mar;100(3):485–492. [PubMed] [Google Scholar]
- Miller C. L., DeWitt C. W. The effect of neonatal thymectomy on antibody responses to histocompatibility antigens in the rat. Cell Immunol. 1974 Aug;13(2):278–287. doi: 10.1016/0008-8749(74)90245-7. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Grumet F. C., McDevitt H. O. Genetic control of the immune response. The effect of thymectomy on the primary and secondary antibody response of mice to poly-L(tyr, glu)-poly-D, L-ala--poly-L-lys. J Exp Med. 1972 Jan;135(1):126–135. doi: 10.1084/jem.135.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Quagliata J. M., Bensinger D. O., Quagliata F. The role of the thymus during the induction of tolerance of a thymus-dependent antigen. J Immunol. 1973 Dec;111(6):1712–1721. [PubMed] [Google Scholar]
- Roelants G. E., Askonas B. A. Immunological B memory in thymus deprived mice. Nat New Biol. 1972 Sep 13;239(89):63–64. doi: 10.1038/newbio239063a0. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Kunz H. W., Gill T. J., 3rd The genetic control of antibody binding constants and specificities in inbred rats. J Immunol. 1974 Nov;113(5):1468–1476. [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Paul W. E., Green I. Histocompatibility-linked immune response gene function in guinea pigs. Specific inhibition of antigen-induced lymphocyte proliferation by alloantisera. J Exp Med. 1972 Nov 1;136(5):1207–1221. doi: 10.1084/jem.136.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair N. R., Elliott E. V. Neonatal thymectomy and the decrease in antigen-sensitivity of the primary response and immunological "memory" systems. Immunology. 1968 Sep;15(3):325–333. [PMC free article] [PubMed] [Google Scholar]
- Sloan B. P., Gill T. J., 3rd Genetic and cellular factors in the immune response. IV. The effect of aggregation on antibody formation and on delayed hypersensitivity in the inbred ACI and F344 strains of rats. J Immunol. 1972 Jan;108(1):26–33. [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Würzburg U., Schütt-Gerowitt H., Rajewsky K. Characterization of an immune response gene in rats. Eur J Immunol. 1973 Dec;3(12):762–766. doi: 10.1002/eji.1830031205. [DOI] [PubMed] [Google Scholar]


