Abstract
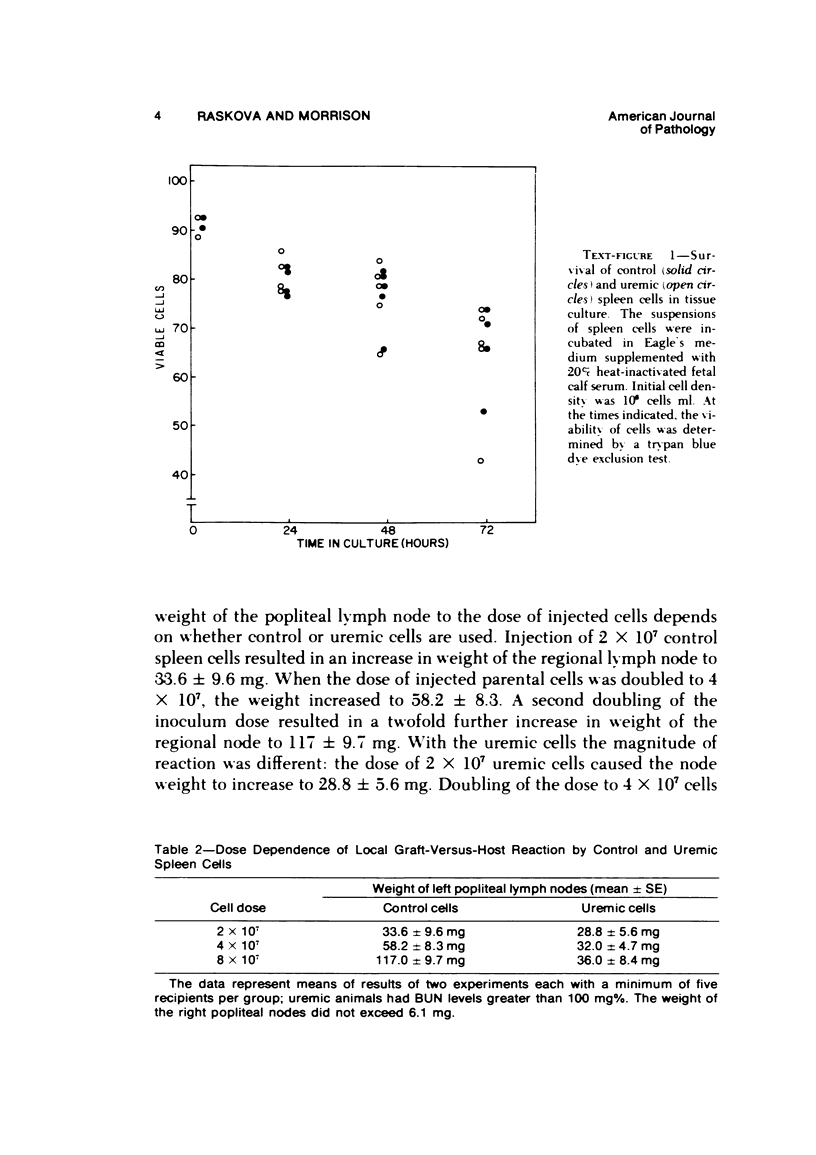
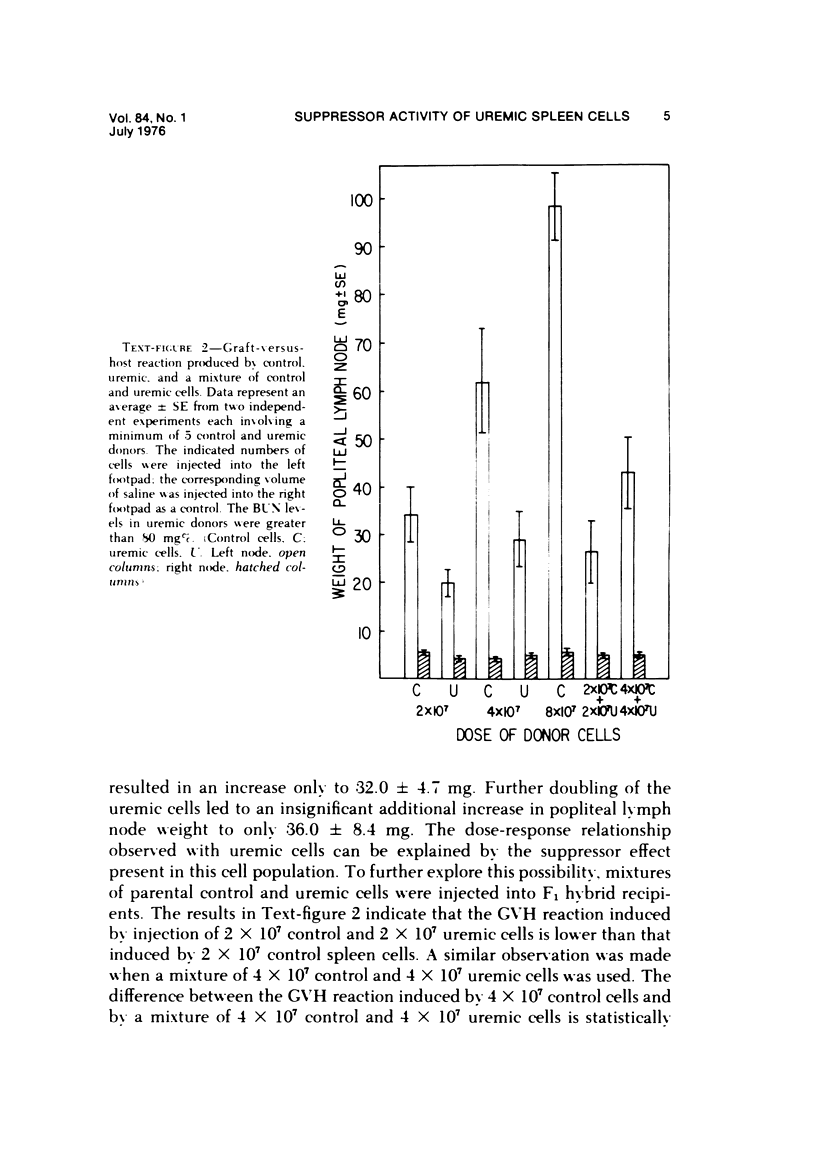
The graft-versus-host (GVH) reactivity of uremic and control spleen cells was studied by popliteal lymph node assay in the rat. The reaction evoked by cells from animals with severe uremia was conspicuously weaker than that evoked by control cells. The magnitude of the GVH reaction induced by control cells was directly proportional to dose, while with the uremic cells the same increases in dose led only to insignificant increases in the strength of the GVH reaction. When mixtures of syngeneic control and uremic cells were used, the GVH reactivity of the control cells was suppressed. The activity of uremic spleen cells can be enhanced (restored) by removal of the sub-population of cells adherent to glass wool. The GVH reaction induced by uremic cells so treated became directly proportional to dose. The removal of the adherent cell population from the uremic spleen cell suspension also led to the disappearance of the suppressor effect in mixtures of control and uremic cells. These results indicate that a decrease in GVH activity of rat uremic spleen cells is due to an increase in suppressor cell activity in the uremic spleen cell population.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALCH H. H. The effect of severe battle injury and of post-traumatic renal failure on resistance to infection. Ann Surg. 1955 Aug;142(2):145–163. doi: 10.1097/00000658-195508000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. B., Glassock R. J., Gleason R., Corson J. M., Merrill J. P. The application of the normal lymphocyte transfer reaction to histocompatibility testing in man. J Clin Invest. 1966 Sep;45(9):1452–1466. doi: 10.1172/JCI105453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M. C., Stites D. P., Fudenberg H. H. Dissociation of responses to phytohaemagglutinin and adult allogeneic lymphocytes in human foetal lymphoid tissues. Nat New Biol. 1973 Feb 28;241(113):279–281. doi: 10.1038/newbio241279a0. [DOI] [PubMed] [Google Scholar]
- DAMMIN G. J., COUCH N. P., MURRAY J. E. Prolonged survival of skin homografts in uremic patients. Ann N Y Acad Sci. 1957 Mar 22;64(5):967–976. doi: 10.1111/j.1749-6632.1957.tb52488.x. [DOI] [PubMed] [Google Scholar]
- Elves M. W., Israëls M. C., Collinge M. An assessment of the mixed leucocyte reaction in renal failure. Lancet. 1966 Mar 26;1(7439):682–685. doi: 10.1016/s0140-6736(66)91628-x. [DOI] [PubMed] [Google Scholar]
- Fink S., Mais R. F. Normal cutaneous sensitivity to endotoxin in azotemia. J Urol. 1970 Nov;104(5):640–641. doi: 10.1016/s0022-5347(17)61799-3. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. In vitro responses of rat lymphocytes following adult thymectomy. I. Rapid decrease and recovery of responses to mitogens and hemiallogeneic cells. J Immunol. 1972 Nov;109(5):1046–1051. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. II. Suppression of mixed lymphocyte reaction by thymus-dependent adherent cells. J Immunol. 1974 Jul;113(1):140–144. [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Gershon R. K., Lance E. M., Kondo K. Immuno-regulatory role of spleen localizing thymocytes. J Immunol. 1974 Feb;112(2):546–554. [PubMed] [Google Scholar]
- Huber H., Pastner D., Dittrich P., Braunsteiner H. In vitro reactivity of human lymphocytes in uraemia--a comparison with the impairment of delayed hypersensitivity. Clin Exp Immunol. 1969 Jul;5(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Kauffman C. A., Manzler A. D., Phair J. P. Cell-mediated immunity in patients on long-term haemodialysis. Clin Exp Immunol. 1975 Oct;22(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE H. S. UREMIA--NATURE'S IMMUNOSUPPRESSIVE DEVICE. Ann Intern Med. 1965 Jan;62:166–170. doi: 10.7326/0003-4819-62-1-166. [DOI] [PubMed] [Google Scholar]
- Merrill J. P. The immunologic capability of uremic patients. Cancer Res. 1968 Jul;28(7):1449–1451. [PubMed] [Google Scholar]
- Raskova J., Morrison A. B. Immunological responses to sheep red blood cells in experimentally induced chronic uremia of rats. Immunol Commun. 1973;2(6):607–614. doi: 10.3109/08820137309022831. [DOI] [PubMed] [Google Scholar]
- STOLOFF I. L., STOUT R., MYERSON R. M., HAVENS W. P., Jr Production of antbody in patients with uremia. N Engl J Med. 1958 Aug 14;259(7):320–323. doi: 10.1056/NEJM195808142590703. [DOI] [PubMed] [Google Scholar]


