Abstract
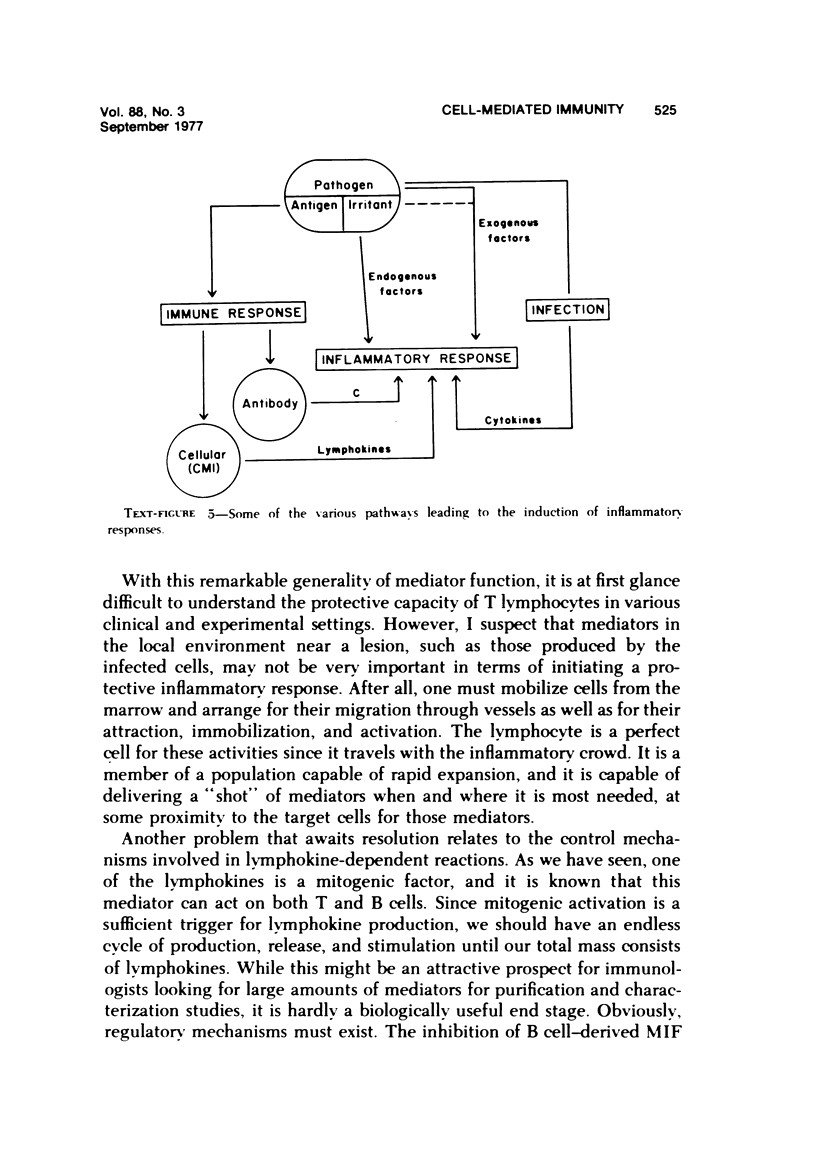
Reactions of cell-mediated immunity fall into two broad categories: those that involve direct participation of intact lymphocytes in the effector mechanism of the reaction and those that involve mediation by soluble lymphocyte-derived factors known as lymphokines. The first kind of reaction is essentially limited to lymphocyte-dependent cytotoxicity, although certain aspects of T cell-B cell cooperation may fall into this category as well. The second category appears to comprise the bulk of the so-called cell-mediated immune response and provides a link between this system and the inflammatory system. Various lymphokines have been shown to exert profound influence upon inflammatory cell metabolism, cell surface properties, patterns of cell migration, and the activation of cells for various biologic activities involved in host defense. Although substantial information is now available about various physicochemical as well as biologic properties of lymphokines, purification and characterization data are as yet too incomplete to allow us to ascribe all of these activities to discrete mediator molecules. Current work involving the development of antibody-based techniques for mediator assay may shed light on this issue. Information on the kinds of cells capable of lymphokine production is now available. Contrary to prior expectation, T cells are not unique in their capacity for lymphokine production. Under appropriate circumstances, B cells and even nonlymphoid cells can do so as well. The unique property of lymphocytes in this regard appears to relate to their ability to respond to certain specialized signals such as specific antigen or an appropriate mitogen. Mediator production per se may represent a general biologic phenomenon. Although lymphokines have been defined mainly in terms of in vitro assays, early speculations about their in vivo importance are proving correct. Evidence for the role of lymphokines comes from studies involving detection of lymphokines in tissues, studies involving injection of exogenous lymphokines, and studies involving suppression of in vivo reactions by various techniques. The use of antilymphokine antibodies has proven useful in the latter kinds of experiments. Work in many laboratories is beginning to relate these findings to clinically relevant situations. A major unsolved problem relates to the regulation and control of lymphokine production and activity. At present only a limited body of information is available on this point. This is a potentially fruitful area for future investigation since it may provide techniques for manipulating the immune system in ways that are clinically useful.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Shevach E. Requirement for T cells in the production of migration inhibitory factor. J Exp Med. 1975 Nov 1;142(5):1306–1311. doi: 10.1084/jem.142.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Stoner G., Gaffney J., Shevach E., Green I. Production of migration inhibitory factor and lymphotoxin by non-T cells. Eur J Immunol. 1975 Mar;5(3):218–220. doi: 10.1002/eji.1830050314. [DOI] [PubMed] [Google Scholar]
- Churchill W. H., Jr, Piessens W. F., Sulis C. A., David J. R. Macrophages activated as suspension cultures with lymphocyte mediators devoid of antigen become cytotoxic for tumor cells. J Immunol. 1975 Sep;115(3):781–786. [PMC free article] [PubMed] [Google Scholar]
- Cohen M. C., Zeschke R., Bigazzi P. E., Yoshida T., Cohen S. Mastocytoma cell migration in vitro: inhibition by MIF-containing supernatants. J Immunol. 1975 May;114(5):1641–1643. [PubMed] [Google Scholar]
- Cohen S., Benacerraf B., McCluskey R. T., Ovary Z. Effect of anticoagulants on delayed hypersensitivity reactions. J Immunol. 1967 Feb;98(2):351–358. [PubMed] [Google Scholar]
- Cohen S. Cell mediated immunity and the inflammatory system. Hum Pathol. 1976 May;7(3):249–264. doi: 10.1016/s0046-8177(76)80036-6. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fisher B., Yoshida T., Bettigole R. E. Serum migration-inhibitory activity in patients with lymphoproliferative diseases. N Engl J Med. 1974 Apr 18;290(16):882–886. doi: 10.1056/NEJM197404182901605. [DOI] [PubMed] [Google Scholar]
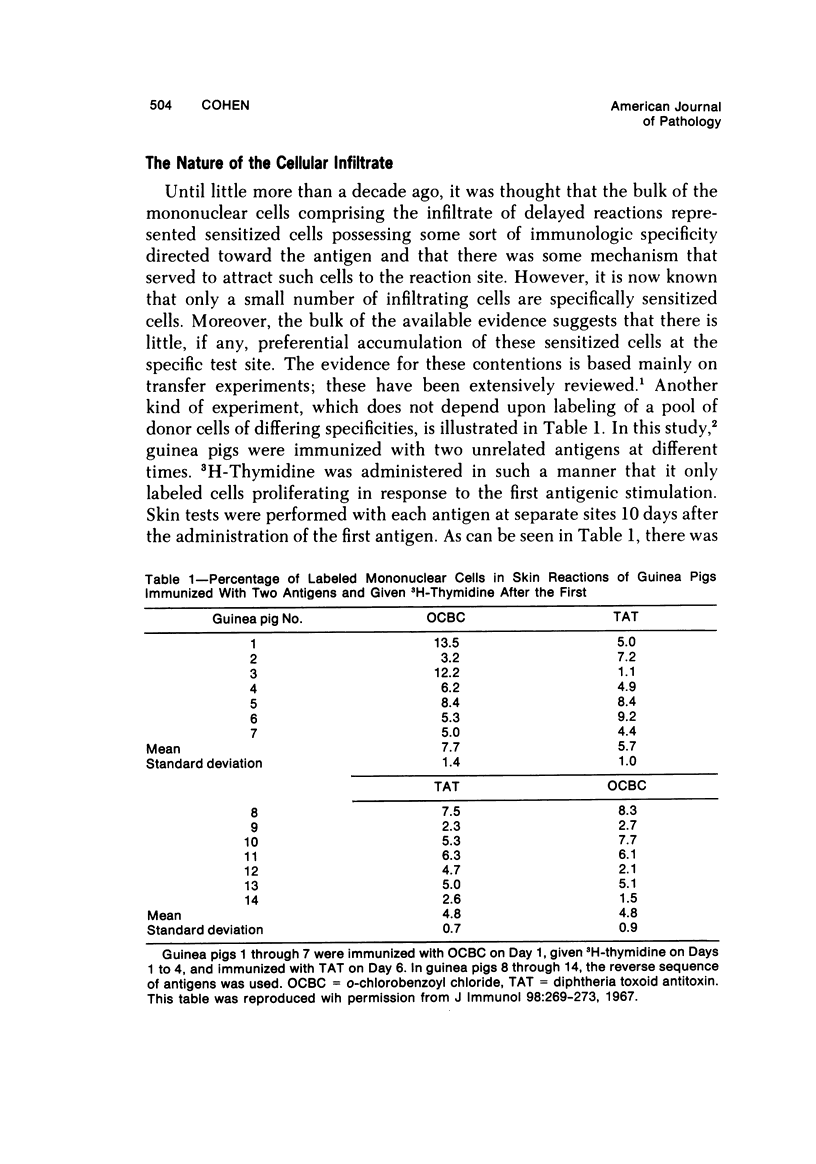
- Cohen S., McCluskey R. T., Benacerraf B. Studies on the specificity of the cellular infiltrate of delayed hypersensitivity reactions. J Immunol. 1967 Feb;98(2):269–273. [PubMed] [Google Scholar]
- Cohen S., Rose N. R., Brown R. C. The appearance of eosinophils during the development of experimental autoimmune thyroiditis in the guinea pig. Clin Immunol Immunopathol. 1974 Jan;2(2):256–265. doi: 10.1016/0090-1229(74)90043-9. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ward P. A. In vitro and in vivo activity of a lymphocyte and immune complex-dependent chemotactic factor for eosinophils. J Exp Med. 1971 Jan 1;133(1):133–146. doi: 10.1084/jem.133.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Ward P. A., Yoshida T., Burek C. L. Biologic activity of extracts of delayed hypersensitivity skin reaction sites. Cell Immunol. 1973 Dec;9(3):363–376. doi: 10.1016/0008-8749(73)90051-8. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M., Johnson R. A., Manseau E. J., Morgan E., Colvin R. B. Morphology of delayed type hypersensitivity reactions in man. I. Quantitative description of the inflammatory response. Lab Invest. 1974 Aug;31(2):111–130. [PubMed] [Google Scholar]
- Epstein L. B., Kreth H. W., Herzenberg L. A. Fluorescence-activated cell sorting of human T and B lymphocytes. II. Identification of the cell type responsible for interferon production and cell proliferation in response to mitogens. Cell Immunol. 1974 Jun;12(3):407–421. doi: 10.1016/0008-8749(74)90097-5. [DOI] [PubMed] [Google Scholar]
- Hammond M. E., Roblin R. O., Dvorak A. M., Selvaggio S. S., Black P. H., Dvorak H. F. MIF-like activity in simian virus 40-transformed 3T3 fibroblast cultures. Science. 1974 Sep 13;185(4155):955–957. doi: 10.1126/science.185.4155.955. [DOI] [PubMed] [Google Scholar]
- Kuratsuji T., Yoshida T., Cohen S. Anti-lymphokine antibody. II. Specificity of biological activity. J Immunol. 1976 Nov;117(5 PT2):1985–1991. [PubMed] [Google Scholar]
- Leber P. D., Milgrom M., Cohen S. Eosinophils in delayed hypersensitivity skin reaction sites. Immunol Commun. 1973;2(6):615–620. doi: 10.3109/08820137309022832. [DOI] [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Rosenstreich D. L., Oppenheim J. J. Induction of lymphokine production by EAC and of blastogenesis by soluble mitogens during human B-cell activation. Nature. 1974 Jun 28;249(460):834–837. doi: 10.1038/249834a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. The effects of anticoagulants and other drugs on cellular and cutaneous reactions to antigen in guinea-pigs with delayed-type hypersensitivity. Immunology. 1965 Sep;9(3):219–234. [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E. Role of monosaccharides in the interaction of two lymphocyte mediators with their target cells. J Immunol. 1976 Mar;116(3):816–820. [PubMed] [Google Scholar]
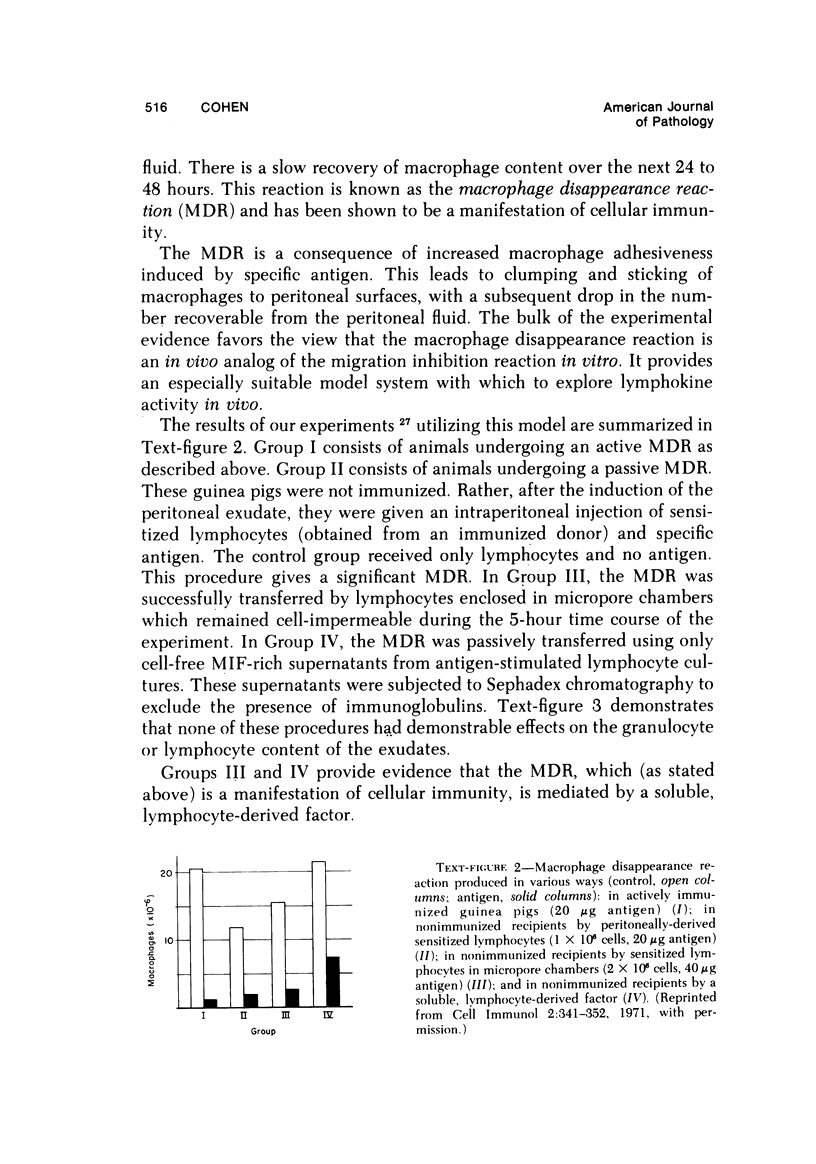
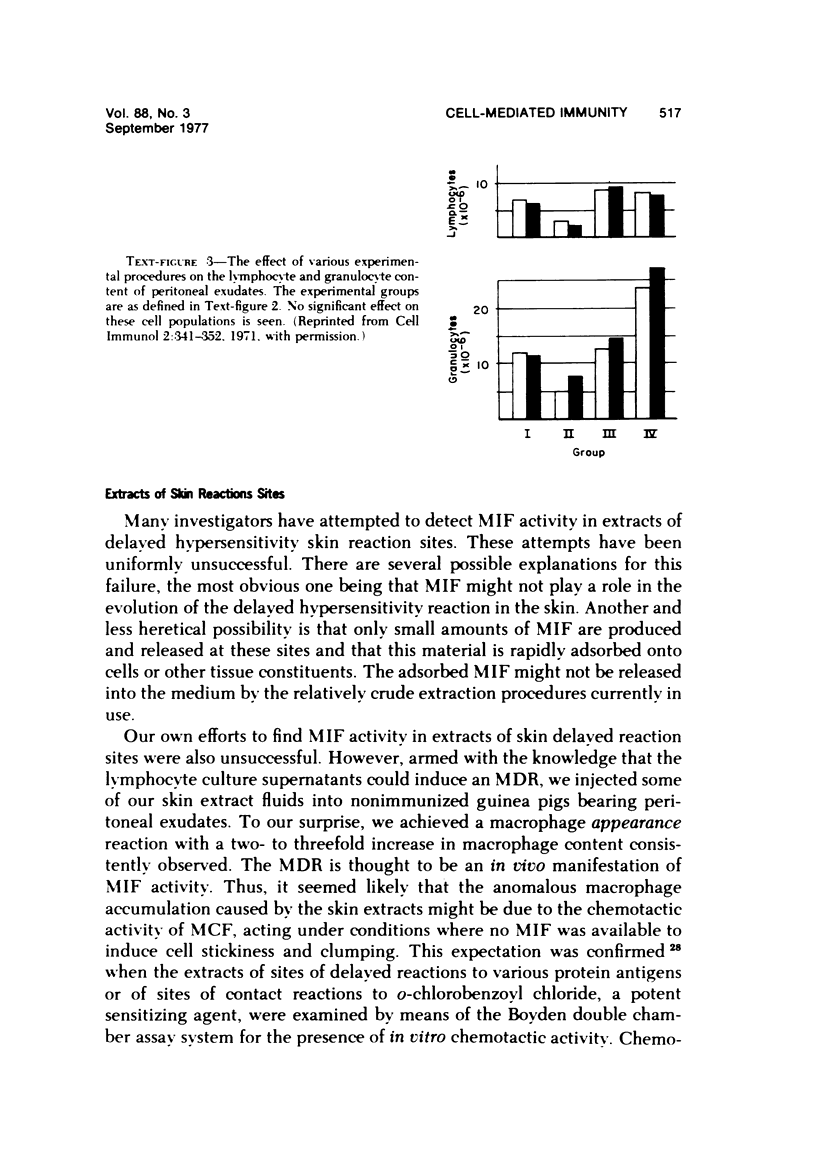
- Sonozaki H., Cohen S. The macrophage disappearance reaction: mediation by a soluble lymphocyte-derived factor. Cell Immunol. 1971 Aug;2(4):341–352. doi: 10.1016/0008-8749(71)90069-4. [DOI] [PubMed] [Google Scholar]
- Sonozaki H., Papermaster V., Yoshida T., Cohen S. Desensitization: effects on cutaneous and peritoneal manifestations of delayed hypersensitivity in relation to lymphokine production. J Immunol. 1975 Dec;115(6):1657–1661. [PubMed] [Google Scholar]
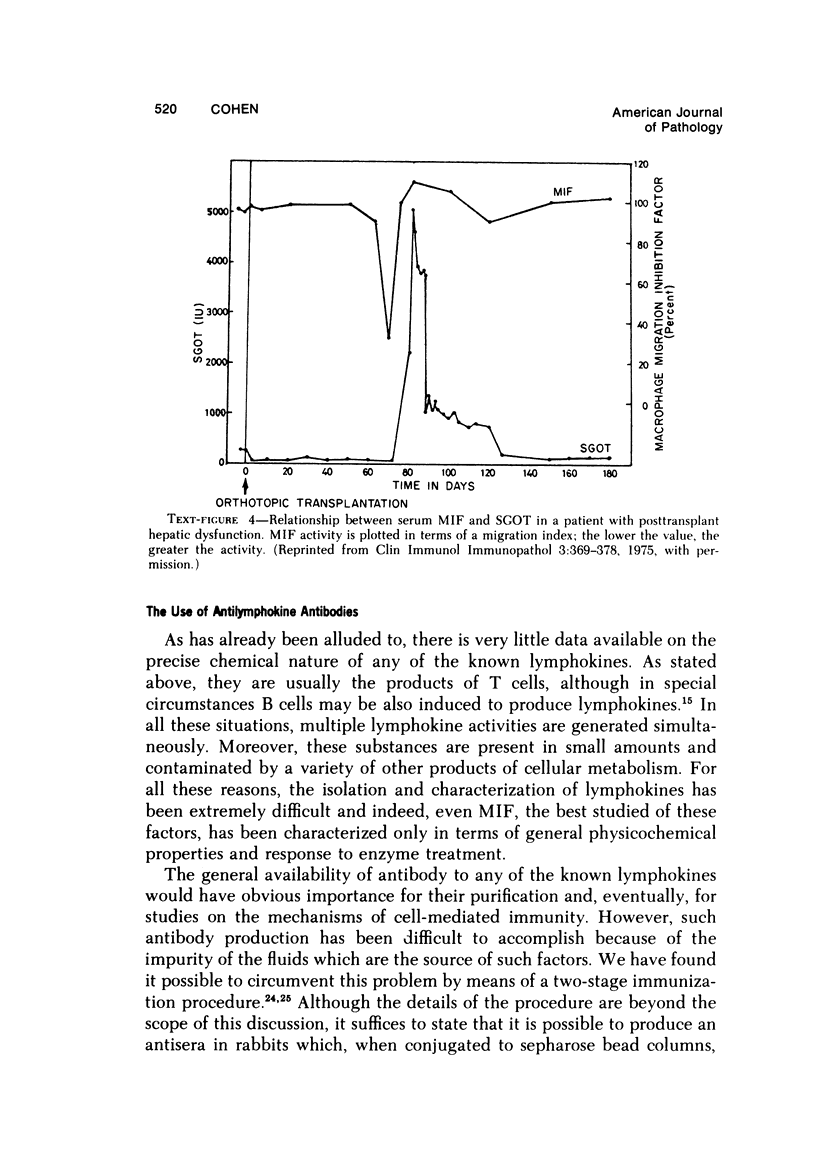
- Torisu M., Yoshida T., Cohen S. Serum migration inhibitory activity in patients with posttransplantation hepatic dysfunction. Clin Immunol Immunopathol. 1975 Jan;3(3):369–376. doi: 10.1016/0090-1229(75)90024-0. [DOI] [PubMed] [Google Scholar]
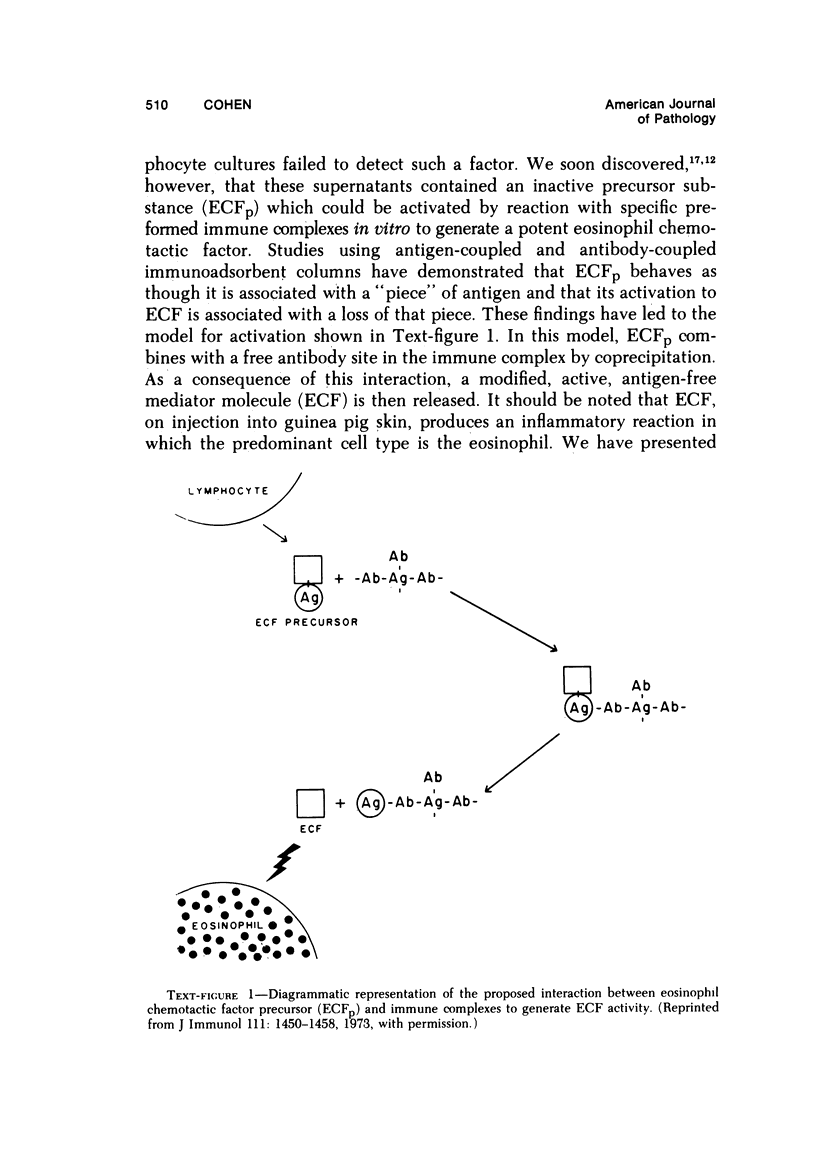
- Torisu M., Yoshida T., Ward P. A., Cohen S. Lymphocyte-derived eosinophil chemotactic factor. II. Studies on the mechanism of activation of the precursor substance by immune complexes. J Immunol. 1973 Nov;111(5):1450–1458. [PubMed] [Google Scholar]
- Wahl S. M., Iverson G. M., Oppenheim J. J. Induction of guinea pig B-cell lymphokine synthesis by mitogenic and nonmitogenic signals to Fc, Ig, and C3 receptors. J Exp Med. 1974 Dec 1;140(6):1631–1645. doi: 10.1084/jem.140.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Benacerraf B., McCluskey R. T., Vassalli P. The effect of intravenous antigen on circulating monocytes in animals with delayed hypersensitivity. J Immunol. 1969 Apr;102(4):804–811. [PubMed] [Google Scholar]
- Yoshida T., Bigazzi P. E., Cohen S. The production of anti-guinea pig lymphokine antibody. J Immunol. 1975 Feb;114(2 Pt 1):688–691. [PubMed] [Google Scholar]
- Yoshida T., Bigazzi P., Cohen S. Biologic and antigenic similarity of virus-induced migration inhibition factor to conventional, lymphocyte-derived migration inhibition factor. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1641–1644. doi: 10.1073/pnas.72.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Cohen S. Lymphokine activity in vivo in relation to circulating monocyte levels and delayed skin reactivity. J Immunol. 1974 Apr;112(4):1540–1547. [PubMed] [Google Scholar]
- Yoshida T., Edelson R., Cohen S., Green I. Migration inhibitory activity in serum and cell supernatants in patients with Sezary syndrome. J Immunol. 1975 Mar;114(3):915–918. [PubMed] [Google Scholar]
- Yoshida T., Kuratsuji T., Takada A., Takada Y., Minowada J., Cohen S. Lymphokine-like factors produced by human lymphoid cell lines with B or T cell surface markers. J Immunol. 1976 Aug;117(2):548–554. [PubMed] [Google Scholar]
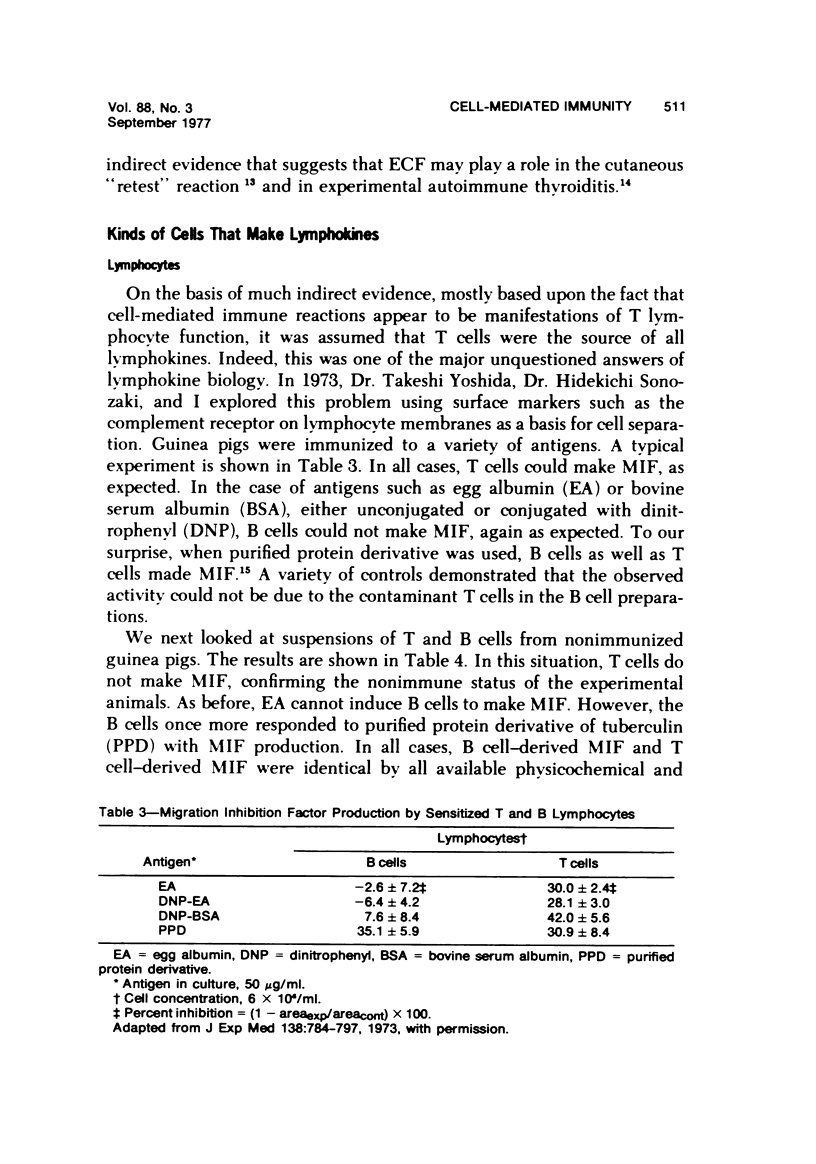
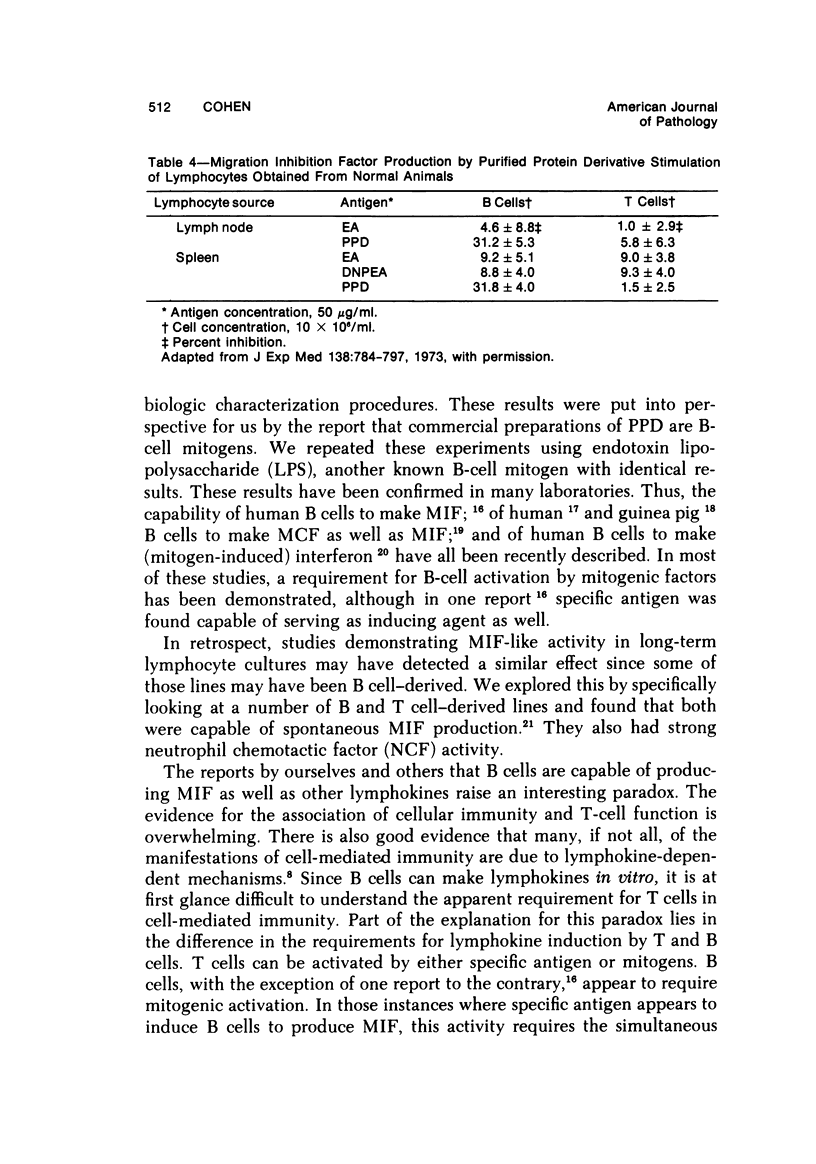
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]


