Abstract
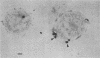
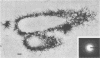
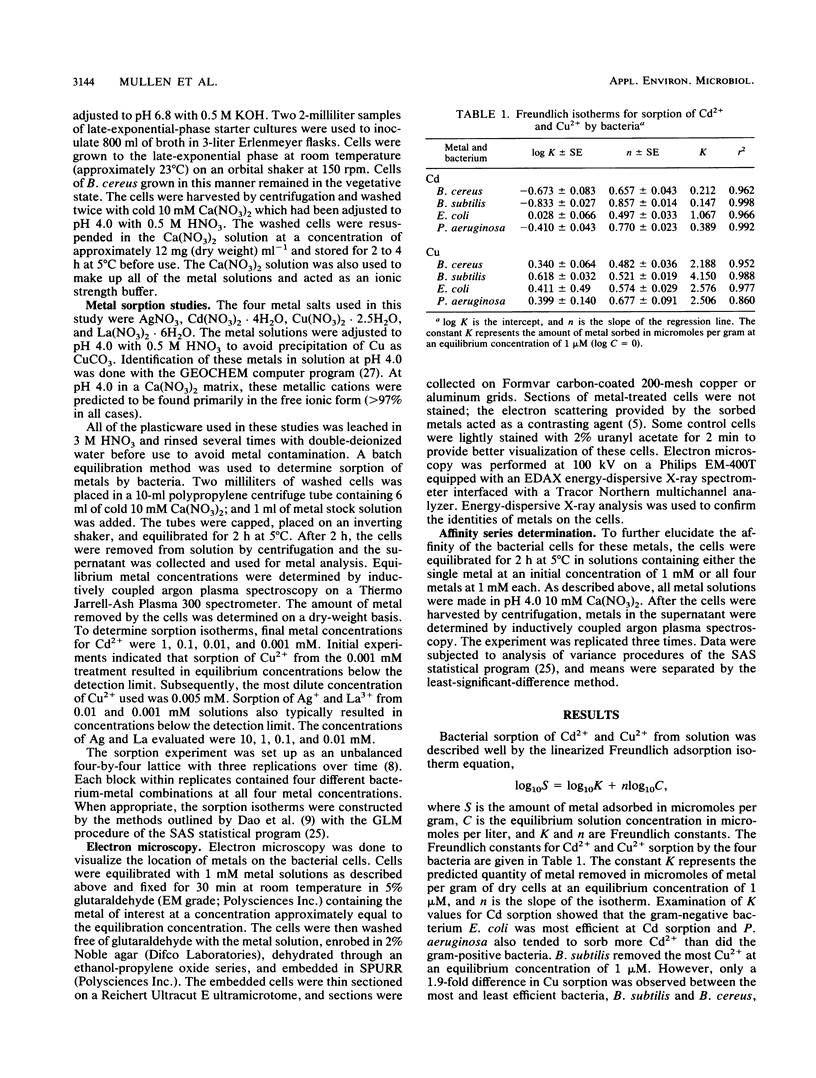
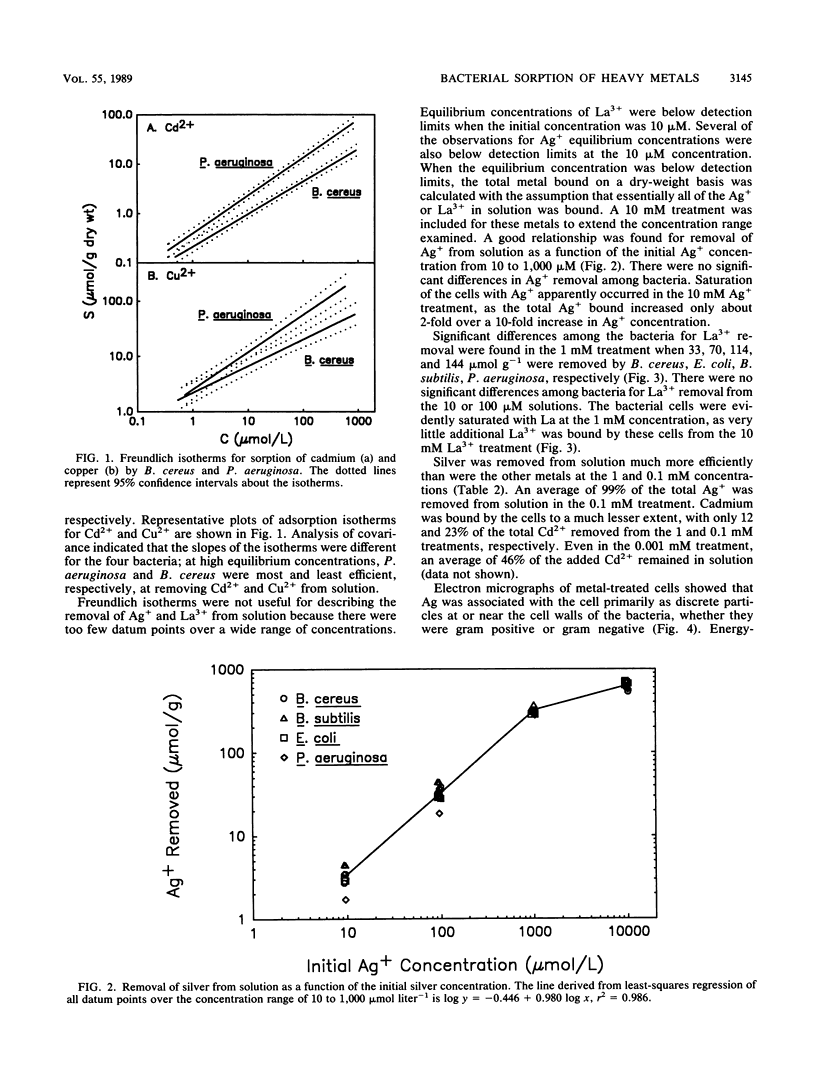
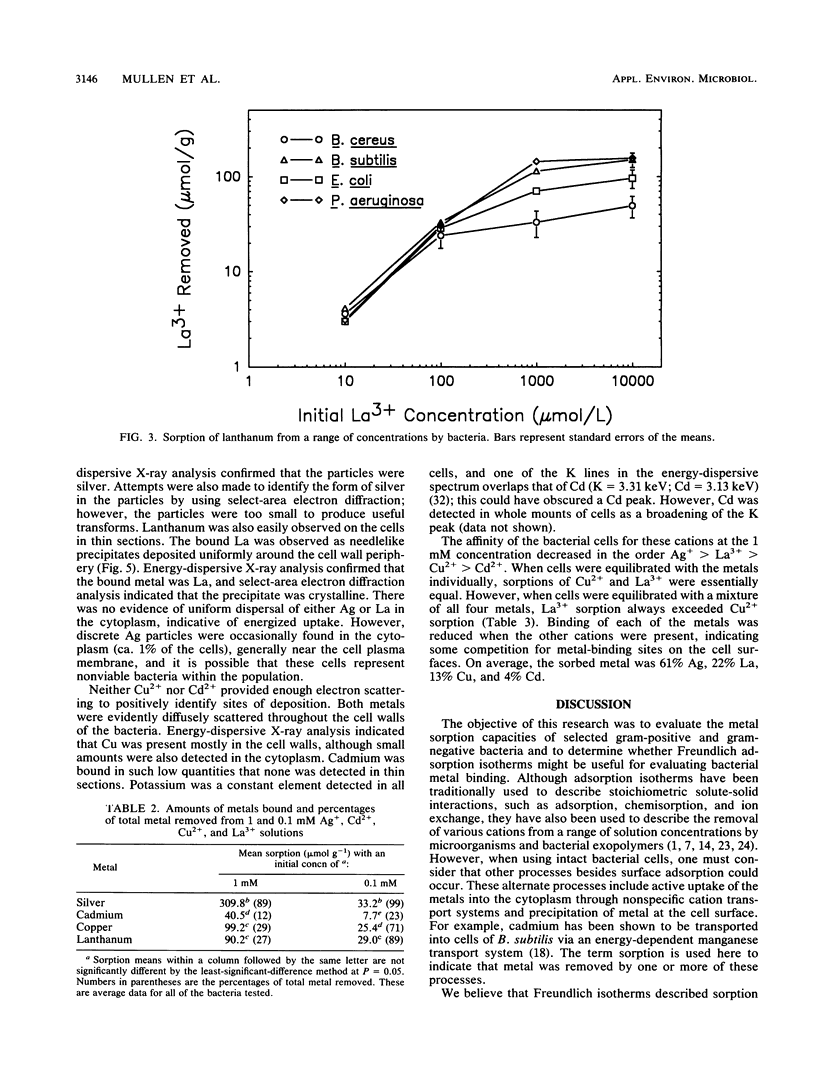
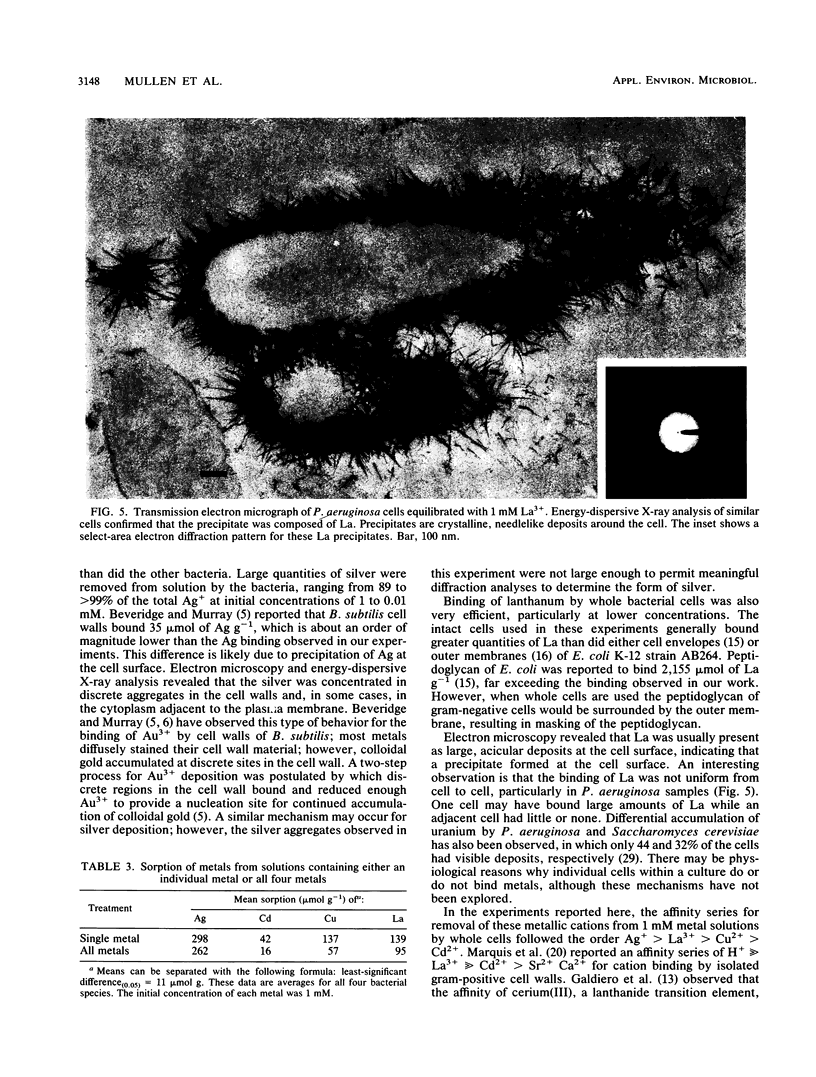
Four bacteria, Bacillus cereus, B. subtilis, Escherichia coli, and Pseudomonas aeruginosa, were examined for the ability to remove Ag+, Cd2+, Cu2+, and La3+ from solution by batch equilibration methods. Cd and Cu sorption over the concentration range 0.001 to 1 mM was described by Freundlich isotherms. At 1 mM concentrations of both Cd2+ and Cu2+, P. aeruginosa and B. cereus were the most and least efficient at metal removal, respectively. Freundlich K constants indicated that E. coli was most efficient at Cd2+ removal and B. subtilis removed the most Cu2+. Removal of Ag+ from solution by bacteria was very efficient; an average of 89% of the total Ag+ was removed from the 1 mM solution, while only 12, 29, and 27% of the total Cd2+, Cu2+, and La3+, respectively, were sorbed from 1 mM solutions. Electron microscopy indicated that La3+ accumulated at the cell surface as needlelike, crystalline precipitates. Silver precipitated as discrete colloidal aggregates at the cell surface and occasionally in the cytoplasm. Neither Cd2+ nor Cu2+ provided enough electron scattering to identify the location of sorption. The affinity series for bacterial removal of these metals decreased in the order Ag greater than La greater than Cu greater than Cd. The results indicate that bacterial cells are capable of binding large quantities of different metals. Adsorption equations may be useful for describing bacterium-metal interactions with metals such as Cd and Cu; however, this approach may not be adequate when precipitation of metals occurs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Meloche J. D., Fyfe W. S., Murray R. G. Diagenesis of metals chemically complexed to bacteria: laboratory formation of metal phosphates, sulfides, and organic condensates in artificial sediments. Appl Environ Microbiol. 1983 Mar;45(3):1094–1108. doi: 10.1128/aem.45.3.1094-1108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988 Apr;34(4):363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Matthews T. H., Streips U. N. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980 Jul;143(1):471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero F., Lembo M., Tufano M. A. Affinity of various cations for Staphylococcus aureus cell-wall. Experientia. 1968 Jan 15;24(1):34–36. doi: 10.1007/BF02136776. [DOI] [PubMed] [Google Scholar]
- Hoyle B. D., Beveridge T. J. Metal binding by the peptidoglycan sacculus of Escherichia coli K-12. Can J Microbiol. 1984 Feb;30(2):204–211. doi: 10.1139/m84-031. [DOI] [PubMed] [Google Scholar]
- Hoyle B., Beveridge T. J. Binding of metallic ions to the outer membrane of Escherichia coli. Appl Environ Microbiol. 1983 Sep;46(3):749–752. doi: 10.1128/aem.46.3.749-752.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek E., Czaban J., Bollag J. M. Sorption of cadmium by microorganisms in competition with other soil constituents. Appl Environ Microbiol. 1982 May;43(5):1011–1015. doi: 10.1128/aem.43.5.1011-1015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube V., Ramamoorthy S., Kushner D. J. Mobilization and accumulation of sediment bound heavy metals by algae. Bull Environ Contam Toxicol. 1979 Apr;21(6):763–770. doi: 10.1007/BF01685502. [DOI] [PubMed] [Google Scholar]
- Marquis R. E., Mayzel K., Carstensen E. L. Cation exchange in cell walls of gram-positive bacteria. Can J Microbiol. 1976 Jul;22(7):975–982. doi: 10.1139/m76-142. [DOI] [PubMed] [Google Scholar]
- Silver S., Misra T. K. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Shumate S. E., Parrott J. R. Microbial Cells as Biosorbents for Heavy Metals: Accumulation of Uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl Environ Microbiol. 1981 Jan;41(1):237–245. doi: 10.1128/aem.41.1.237-245.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin J. M., Cooper D. G., Neufeld R. J. Uptake of Metal Ions by Rhizopus arrhizus Biomass. Appl Environ Microbiol. 1984 Apr;47(4):821–824. doi: 10.1128/aem.47.4.821-824.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. G., Flemming C. A., Ferris F. G., Beveridge T. J., Bailey G. W. Physicochemical interaction of Escherichia coli cell envelopes and Bacillus subtilis cell walls with two clays and ability of the composite to immobilize heavy metals from solution. Appl Environ Microbiol. 1989 Nov;55(11):2976–2984. doi: 10.1128/aem.55.11.2976-2984.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]