Abstract
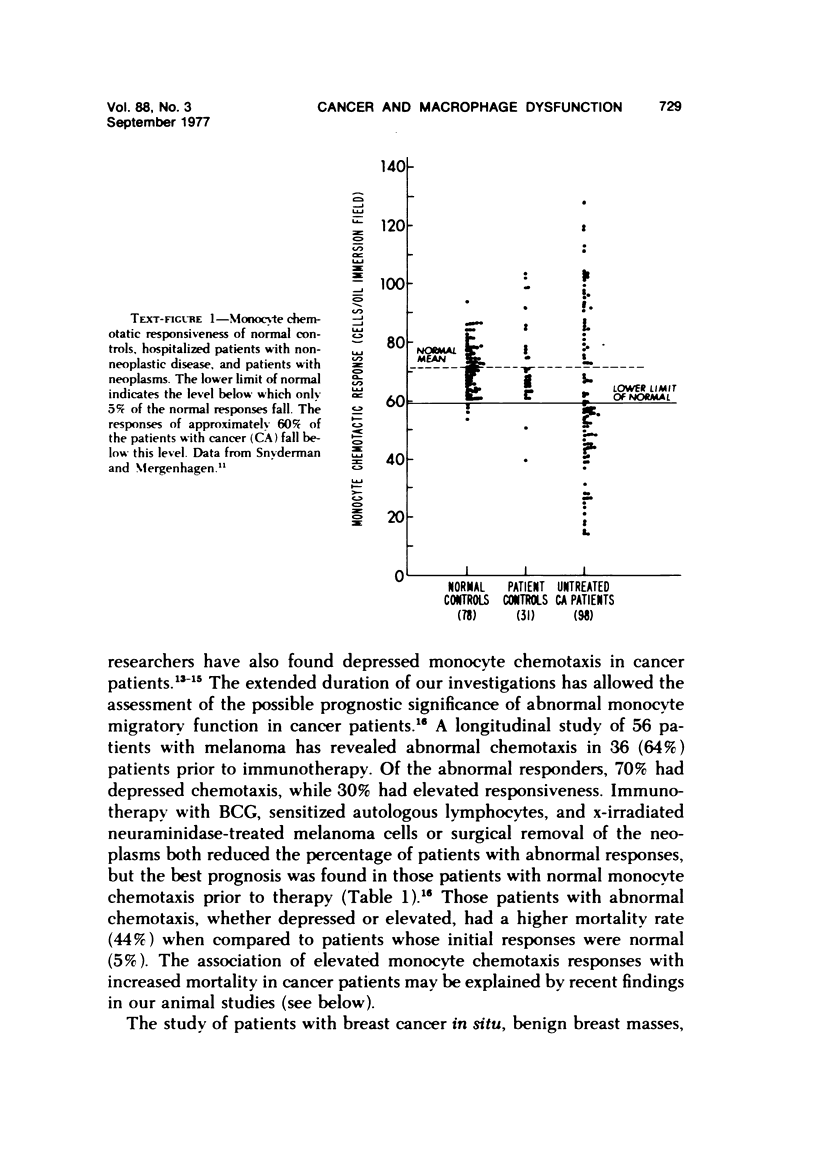
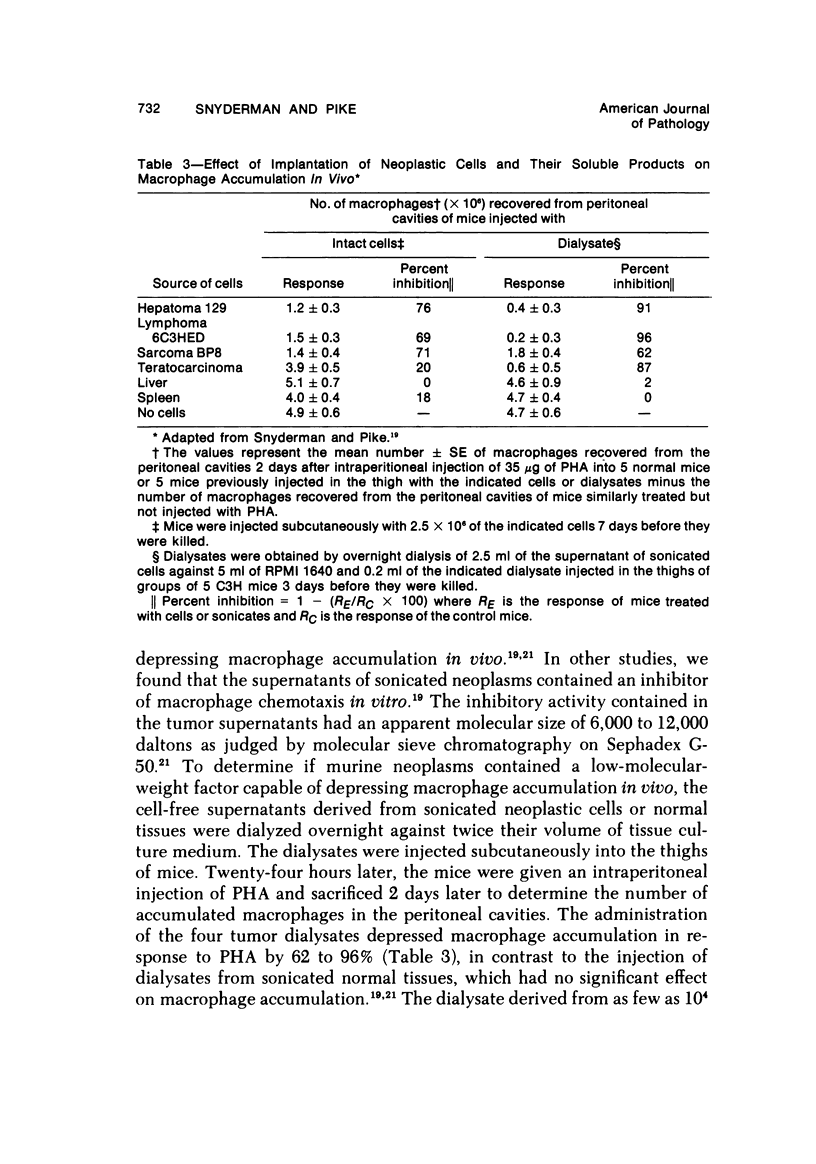
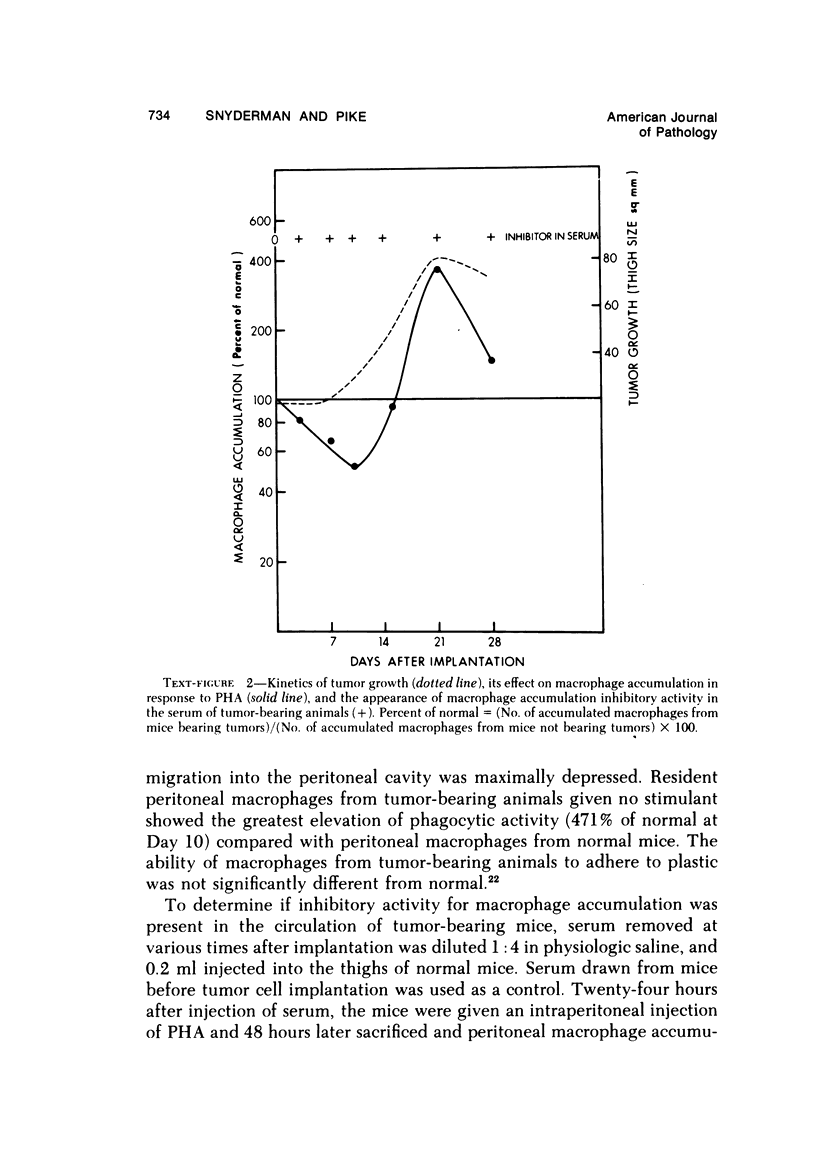
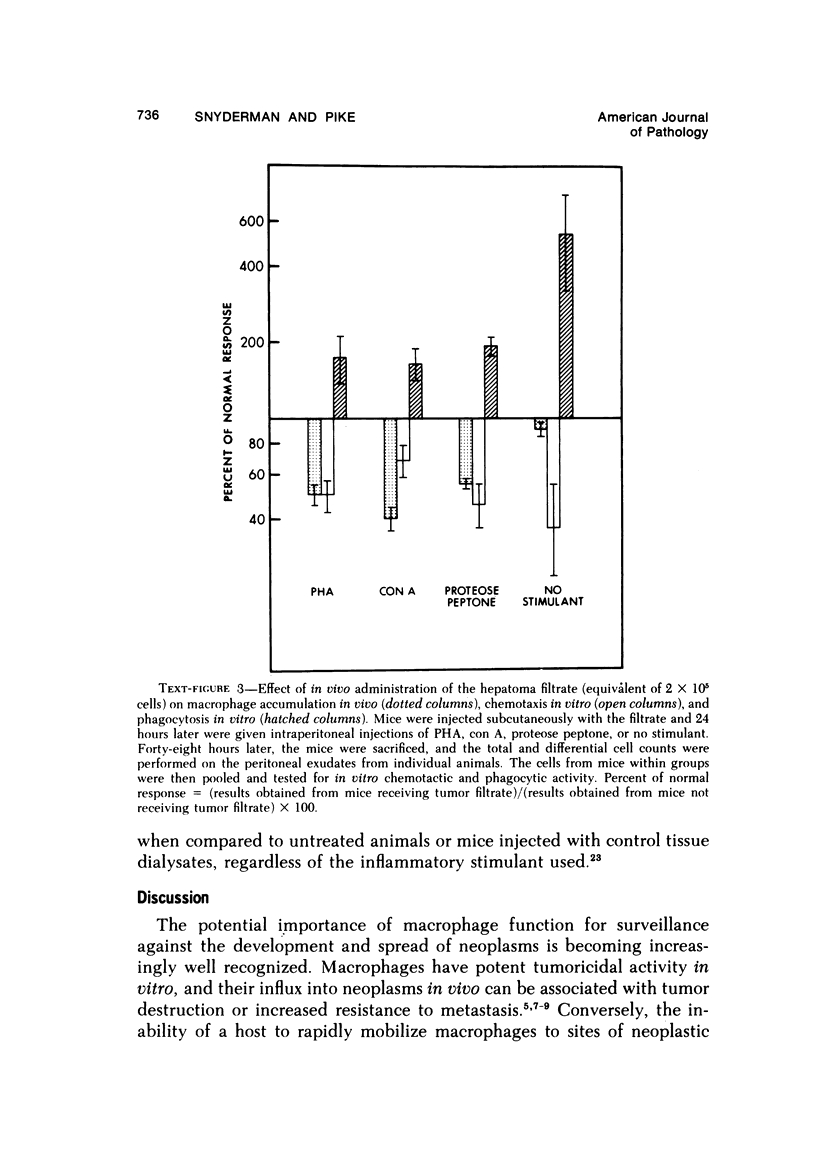
There is considerable evidence to suggest that macrophages participate in host resistance to the development and spread of cancer. We have, therefore, studied monocytemacrophage function in humans and animals with neoplasms. Approximately 60% of patients with various types of cancer were found to have abnormal monocyte chemotactic responsiveness in vitro, and abnormal chemotaxis was an indicator of poor prognosis in patients with melanoma. By studying patients before and after surgery, it was found that abnormal chemotactic responses normalized within weeks after removal of malignant tumors, indicating that a neoplasm itself might affect the host's monocyte chemotactic responsiveness. Subsequent studies using transplantable neoplasms in mice substantiated this hypothesis in that macrophage accumulation in vivo as well as macrophage chemotactic responsiveness in vitro was depressed in animals during the early phases of tumor growth. This depression of macrophage function could be attributed to a low-molecular-weight factor contained in murine neoplasms, which when given to normal mice was extremely potent in depressing peritoneal macrophage accumulation and chemotaxis but, paradoxically, enhanced phagocytosis. The serum of tumor-bearing mice also contained potent inhibitory activity for macrophage accumulation. In contrast to the effects on macrophages, granulocyte accumulation in vivo and chemotaxis in vitro was not depressed by the presence of a neoplasm or the administration of the factor from neoplasms. By releasing factors which depress macrophage migratory function, neoplasms may protect themselves from immunologically mediated host destruction during the early phases of tumor growth.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boetcher D. A., Leonard E. J. Abnormal monocyte chemotactic response in cancer patients. J Natl Cancer Inst. 1974 Apr;52(4):1091–1099. doi: 10.1093/jnci/52.4.1091. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Evans R. Macrophages in syngeneic animal tumours. Transplantation. 1972 Oct;14(4):468–473. doi: 10.1097/00007890-197210000-00011. [DOI] [PubMed] [Google Scholar]
- Hausman M. S., Brosman S., Snyderman R., Mickey M. R., Fahey J. Defective monocyte function in patients with genitourinary carcinoma. J Natl Cancer Inst. 1975 Nov;55(5):1047–1054. doi: 10.1093/jnci/55.5.1047. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. The role of macrophages in defense against neoplastic disease. Adv Cancer Res. 1974;20:131–163. doi: 10.1016/s0065-230x(08)60110-4. [DOI] [PubMed] [Google Scholar]
- Norman S. J., Sorkin E. Cell-specific defect in monocyte function during tumor growth. J Natl Cancer Inst. 1976 Jul;57(1):135–140. doi: 10.1093/jnci/57.1.135. [DOI] [PubMed] [Google Scholar]
- Normann S. J., Sorkin E. Inhibition of macrophage chemotaxis by neoplastic and other rapidly proliferating cells in vitro. Cancer Res. 1977 Mar;37(3):705–711. [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Prehn R. T., Prehn L. M. Pathobiology of neoplasia. A teaching monograph. Am J Pathol. 1975 Sep;80(3):529–550. [PMC free article] [PubMed] [Google Scholar]
- Rubin R. H., Cosimi A. B., Goetzl E. J. Defective human mononuclear leukocyte chemotaxis as an index of host resistance to malignant melanoma. Clin Immunol Immunopathol. 1976 Nov;6(3):376–388. doi: 10.1016/0090-1229(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Hayden M., Langley S., Kaliss N., Smith M. R. Antibody-mediated suppression of grafted lymphoma. III. Evaluation of the role of thymic function, non-thymus-derived lymphocytes, macrophages, platelets, and polymorphonuclear leukocytes in syngeneic and allogeneic hosts. J Immunol. 1975 Apr;114(4):1255–1263. [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. An inhibitor of macrophage chemotaxis produced by neoplasms. Science. 1976 Apr 23;192(4237):370–372. doi: 10.1126/science.946556. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., Blaylock B. L., Weinstein P. Effects of neoplasms on inflammation: depression of macrophage accumulation after tumor implantation. J Immunol. 1976 Mar;116(3):585–589. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C., McCarley D., Lang L. Quantification of mouse macrophage chemotaxis in vitro: role of C5 for the production of chemotactic activity. Infect Immun. 1975 Mar;11(3):488–492. doi: 10.1128/iai.11.3.488-492.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Seigler H. F., Meadows L. Abnormalitieis of monocyte chemotaxis in patients with melanoma: effects of immunotherapy and tumor removal. J Natl Cancer Inst. 1977 Jan;58(1):37–41. doi: 10.1093/jnci/58.1.37. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Meltzer M. S. Depressed chemotactic responses in vitro of peritoneal macrophages from tumor-bearing mice. J Natl Cancer Inst. 1976 Oct;57(4):847–852. doi: 10.1093/jnci/57.4.847. [DOI] [PubMed] [Google Scholar]
- Zbar B., Wepsic H. T., Rapp H. J., Stewart L. C., Borsos T. Two-step mechanism of tumor graft rejection in syngeneic guinea pigs. II. Initiation of reaction by a cell fraction containing lymphocytes and neutrophils. J Natl Cancer Inst. 1970 Mar;44(3):701–717. [PubMed] [Google Scholar]


