Abstract
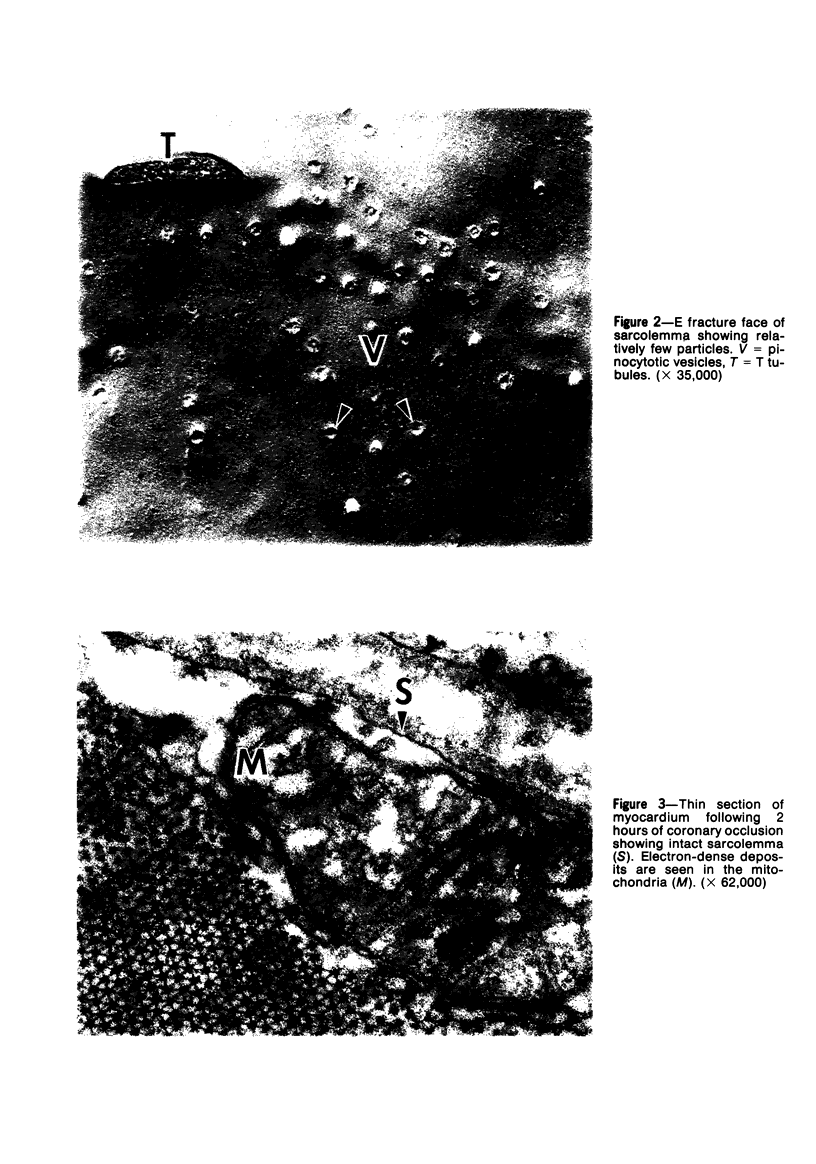
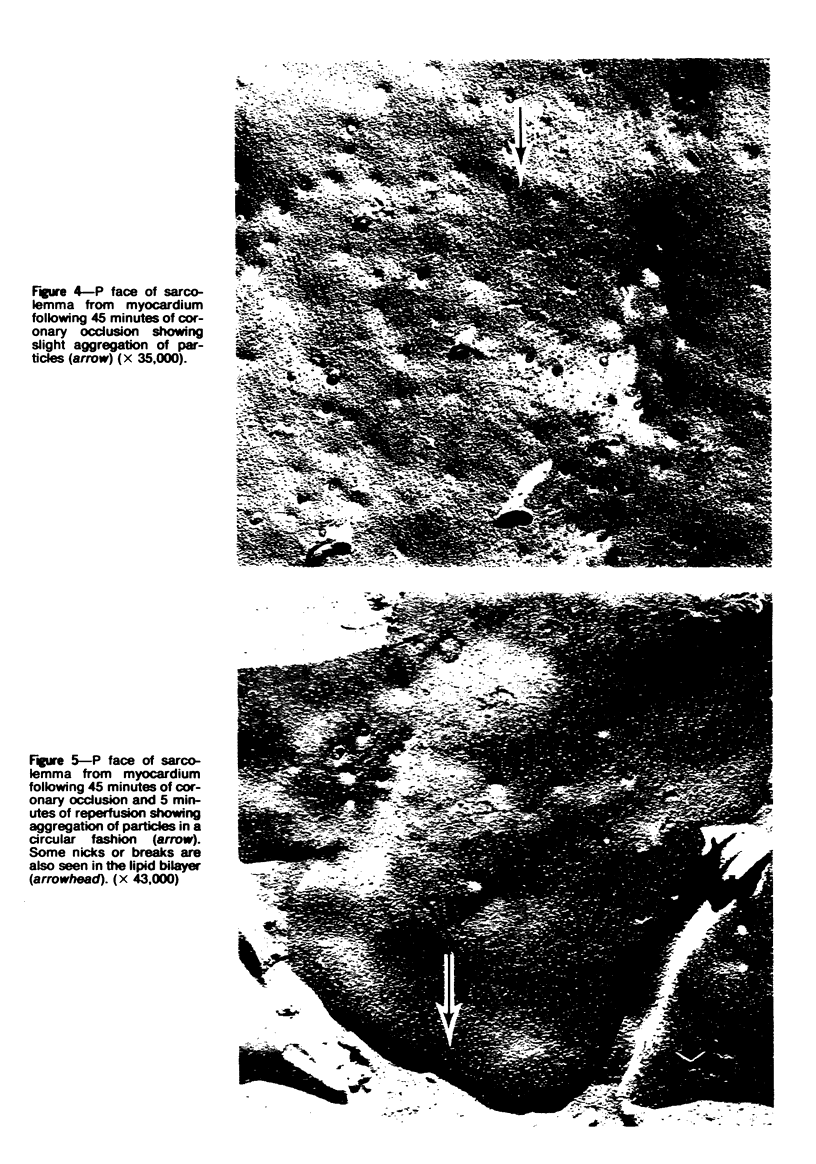
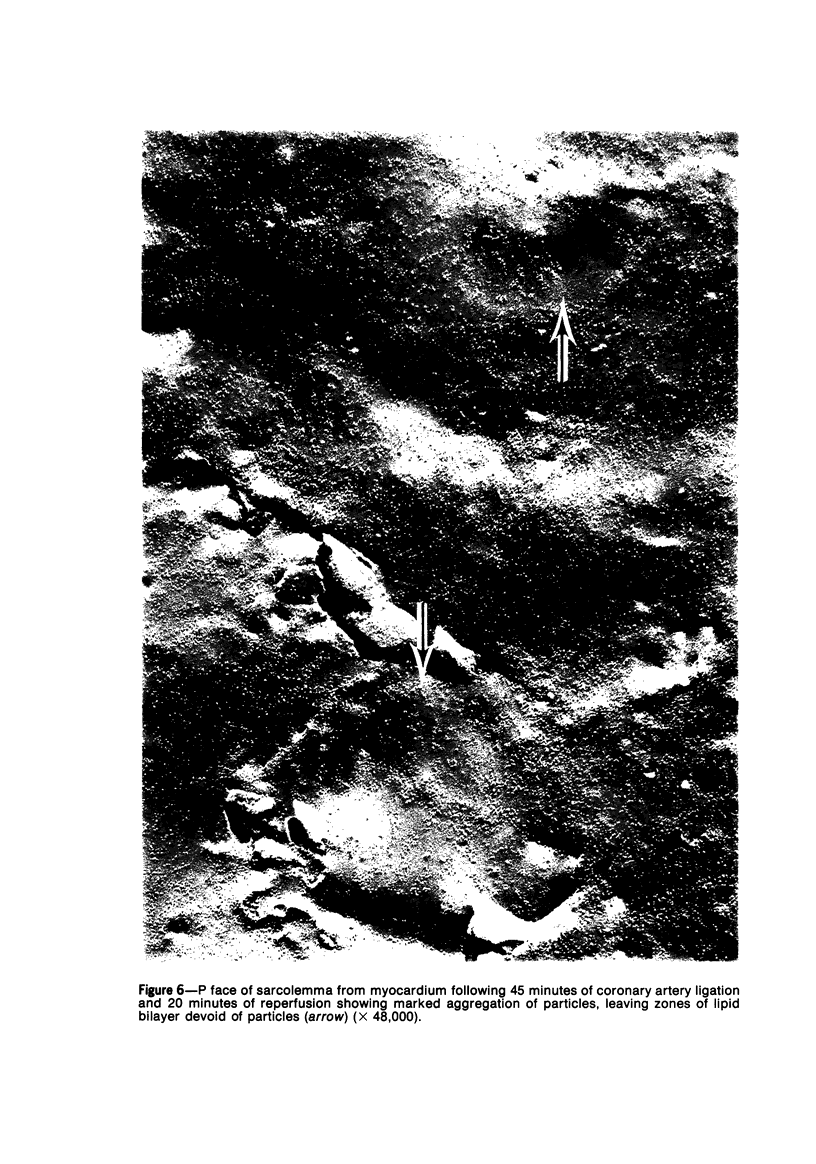
Ultrastructural alterations in the sarcolemma of ischemic myocardium were studied with the freeze-fracture technique. In normal myocardial sarcolemma, the P fracture face contained many intramembranous particles which were randomly distributed, while the E fracture face had few intramembranous particles; no structural abnormalities were seen within the lipid bilayer on either face. Myocardium ischemic for 45 minutes displayed no, or only slight, aggregation of intramembranous particles, but upon reperfusion for 5 to 20 minutes, the particles became significantly aggregated in the P face. Scattered nicks within the lipid bilayer were observed on both fracture faces. Intramembranous particles were similarly aggregated in myocardium ischemic for 2 hours; however, the number of nicks were greatly increased on the P and E fracture faces. These structural alterations, which were undetected in thin sections, are likely to be associated with altered function in the sarcolemma of ischemic myocardium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONN H. L., Jr, WOOD J. C., MORALES G. S. Rate of change in myocardial glycogen and lactic acid following arrest of coronary circulation. Circ Res. 1959 Sep;7:721–727. doi: 10.1161/01.res.7.5.721. [DOI] [PubMed] [Google Scholar]
- Chevalier J., Bourguet J., Hugon J. S. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 1974;152(2):129–140. doi: 10.1007/BF00224690. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., Duggan J., Hackett R. L. Plasma membrane changes in freeze-fractured rat kidney cortex following renal ischemia. Lab Invest. 1976 Jul;35(1):63–70. [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Herdson P. B., Kaltenbach J. P., Jennings R. B. Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol. 1969 Dec;57(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K. Protein-liposome interactions and their relevance to the structure and function of cell membranes. Mol Cell Biochem. 1976 Feb 25;10(3):171–190. doi: 10.1007/BF01731688. [DOI] [PubMed] [Google Scholar]
- McIntyre J. A., Gilula N. B., Karnovsky M. J. Cryoprotectant-induced redistribution of intramembranous particles in mouse lymphocytes. J Cell Biol. 1974 Jan;60(1):192–203. doi: 10.1083/jcb.60.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]