Abstract
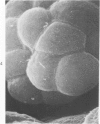
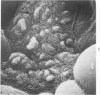
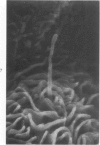
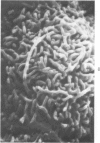
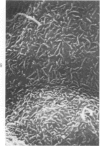
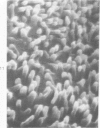
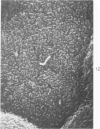
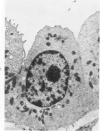
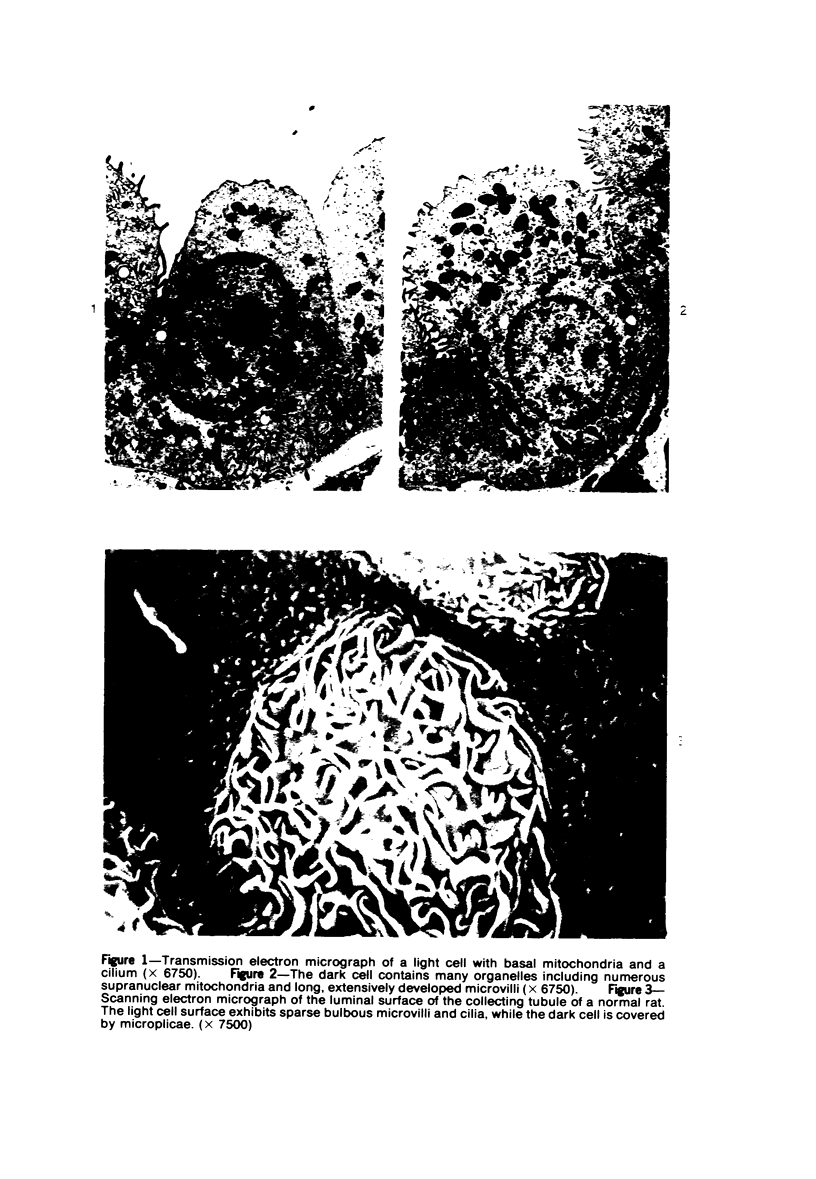
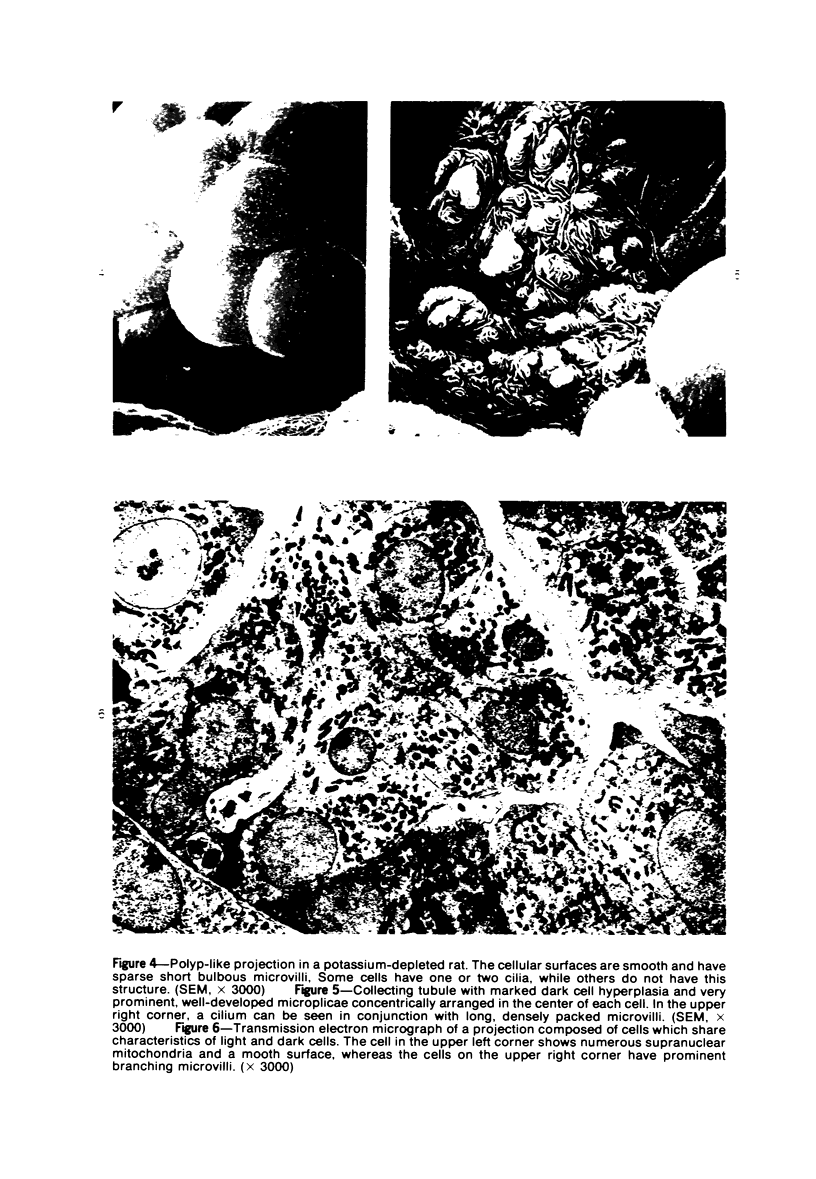
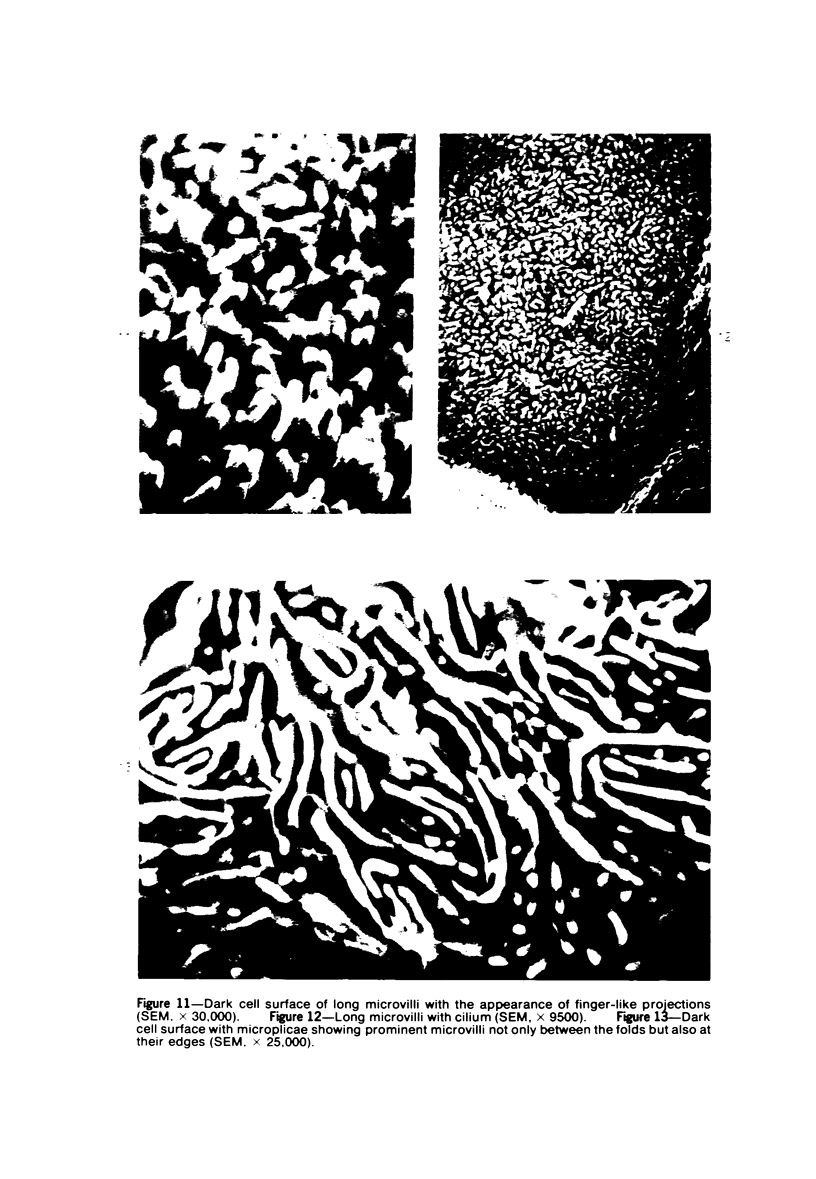
The luminal surface of collecting tubule cells in the inner stripe of the renal medulla in normal and potassium-depleted rats was studied by scanning electron microscopy. In normal rats the luminal surfaces were of two types. One cell type was sparsely covered with small bulbous microvilli and had either a single or double cilium. This type corresponds to the light cell seen in transmission electron microscopy. The second cell type was covered by prominent microplicae and represents the dark cell observed in transmission electron microscopy. In potassium-depleted animals, numerous cells with a morphologic appearance of intermediate forms were identified. By scanning electron microscopy, the luminal surface of these cells was covered by a mixed population of villi and microplicae in different stages of development and often showed cilia, which were previously considered to exist only on light cells. On the basis of these morphologic findings, we conclude that the dark and light cells are not different cell types but rather represent different forms of a single type of cell.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M., Porter K. R. A scanning electron microscopic study of the nephron. Am J Anat. 1974 May;140(1):81–115. doi: 10.1002/aja.1001400107. [DOI] [PubMed] [Google Scholar]
- Bohman S. O. The ultrastructure of the rat renal medulla as observed after improved fixation methods. J Ultrastruct Res. 1974 Jun;47(3):329–360. doi: 10.1016/s0022-5320(74)90014-8. [DOI] [PubMed] [Google Scholar]
- Bulger R. E., Siegel F. L., Pendergrass R. Scanning and transmission electron microscopy of the rat kidney. Am J Anat. 1974 Apr;139(4):483–501. doi: 10.1002/aja.1001390403. [DOI] [PubMed] [Google Scholar]
- CLARK S. L., Jr Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957 May 25;3(3):349–362. doi: 10.1083/jcb.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. D., Bulger R. E., Trump B. F. The ultrastructure of the functioning kidney. Lab Invest. 1967 Feb;16(2):220–246. [PubMed] [Google Scholar]
- Hagége J., Gabe M., Richet G. Scanning of the apical pole of distal tubular cells under differing acid-base conditions. Kidney Int. 1974 Feb;5(2):137–146. doi: 10.1038/ki.1974.18. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD M. K., SABOUR M. S., LAMBIE A. T., ROBSON J. S. The nephropathy of experimental potassium deficiency. An electron microscopic study. Q J Exp Physiol Cogn Med Sci. 1962 Jul;47:262–272. doi: 10.1113/expphysiol.1962.sp001606. [DOI] [PubMed] [Google Scholar]
- MUEHRCKE R. C., ROSEN S. HYPOKALEMIC NEPHROPATHY IN RAT AND MAN: A LIGHT AND ELECTRON MICROSCOPIC STUDY. Lab Invest. 1964 Nov;13:1359–1373. [PubMed] [Google Scholar]
- Myers C. E., Bulger R. E., Tisher C. C., Trump B. F. Human ultrastructure. IV. Collecting duct of healthy individuals. Lab Invest. 1966 Dec;15(12):1921–1950. [PubMed] [Google Scholar]
- OLIVER J., MACDOWELL M., WELT L. G., HOLLIDAY M. A., HOLLANDER W., Jr, WINTERS R. W., WILLIAMS T. F., SEGAR W. E. The renal lesions of electrolyte imbalance. I. The structural alterations in potassium-depleted rats. J Exp Med. 1957 Oct 1;106(4):563–574. doi: 10.1084/jem.106.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet G., Hagege J., Gabe M. Corrélations entre les transferts de bicarbonate et la morphologie du segment terminal in nephron chez le rat. Nephron. 1970;7(5):413–429. doi: 10.1159/000179842. [DOI] [PubMed] [Google Scholar]
- SPARGO B. Kidney changes in hypokalemic alkalosis in the rat. J Lab Clin Med. 1954 May;43(5):802–814. [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Ordónez N. G., Bortz S. L., Spargo B. H. Zonal changes in renal structure and phospholipid metabolism in potassium-deficient rats. Lab Invest. 1976 Feb;34(2):115–124. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]