Abstract
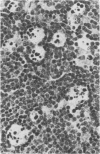
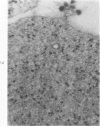
Ultrastructural, histopathologic, and virologic studies of adult hamsters infected with virulent Venezuelan equine encelphalomyelitis (VEE) virus (Subtype I-B) demonstrated precise chronologic and topographic progression of lesions and viral replication in extraneural sites. Thymus contained the earliest lesions and the highest initial and subsequent viral titers. No particular cytotropism was observed as highly efficient viral replication and severe cytonecrosis proceded. Early cortical necrosis of splenic periarteriolar lymphocytic sheath was followed by lymphoblastoid repopulation of the peripheral zone. Massive bone marrow necrosis was accompained by ultrastructural evidence of VEE viral particle production in reticulum cells, rubricytes, myeloid cells, lymphoblastoid cells, and megakaryocytes. Speed, efficiency, destructiveness, and relative sensitivity of virtually all lymphoreticular and hematopoetic cells were hallmarks of virulent VEE infection in the hamster.
Full text
PDF












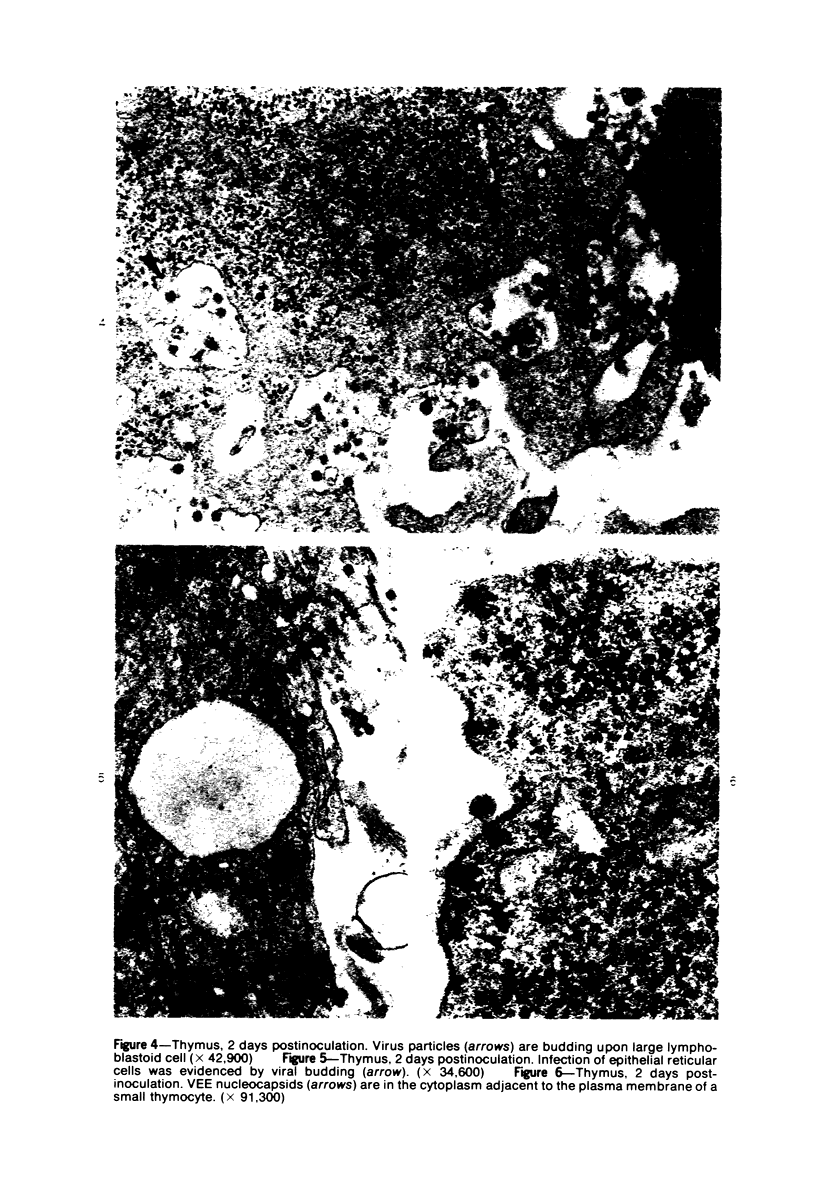
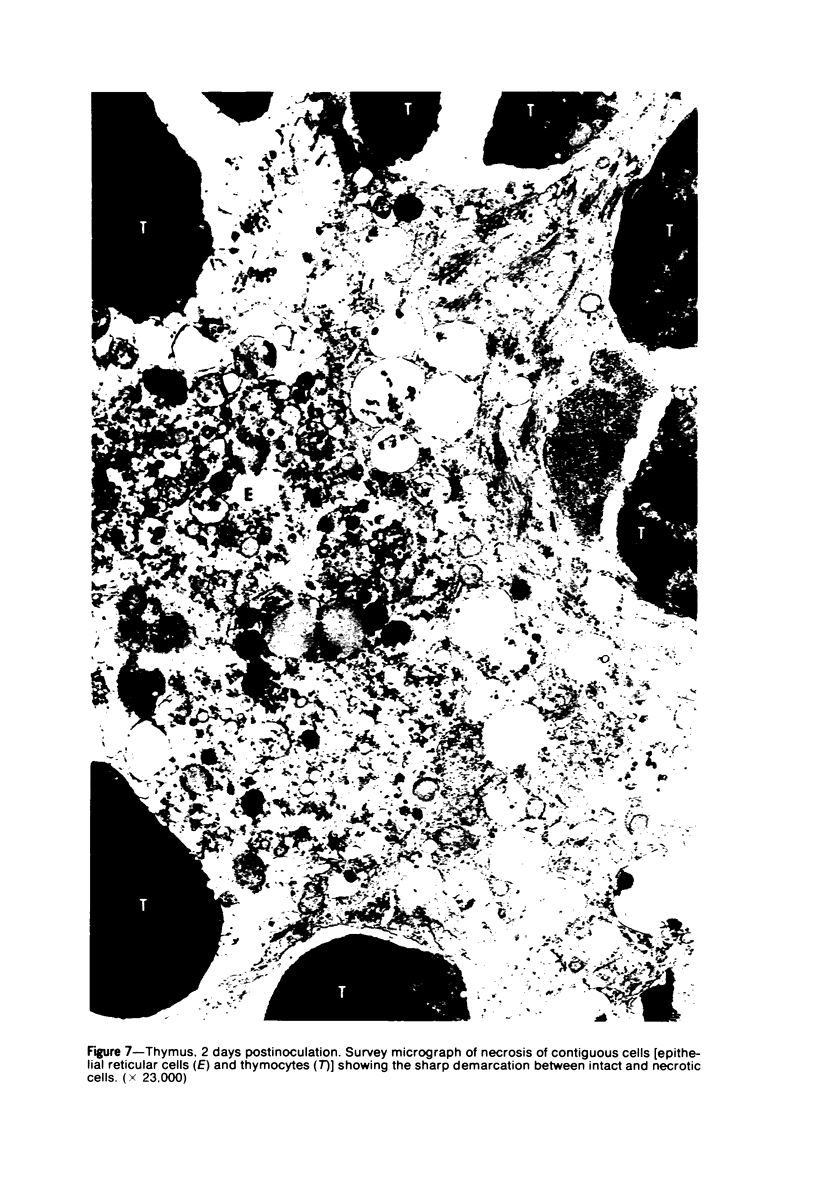

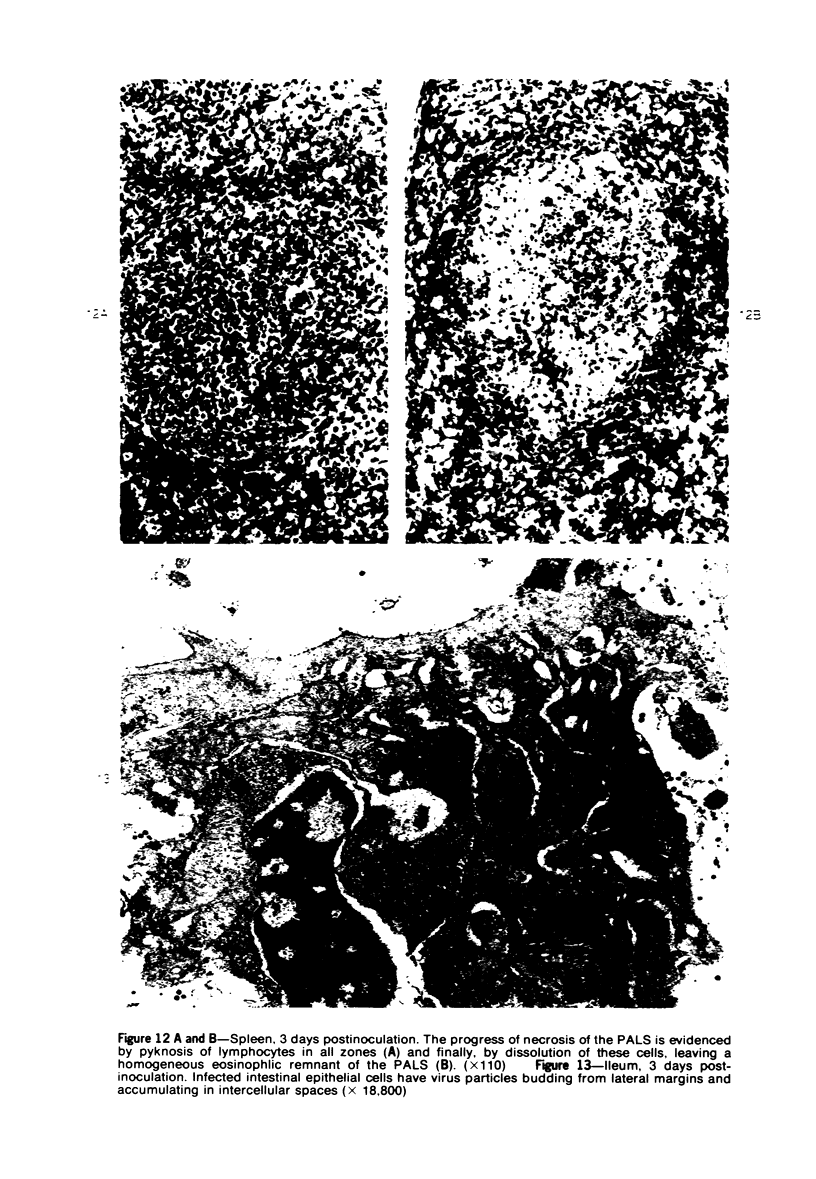
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin F. J., Scherer W. F. Studies of viral virulence. I. Growth and histopathology of virulent and attenuated strains of Venezuelan encephalitis virus in hamsters. Am J Pathol. 1971 Feb;62(2):195–210. [PMC free article] [PubMed] [Google Scholar]
- Chappell W. A., Sasso D. R., Toole R. F., Monath T. P. Labile serum factor and its effect on arbovirus neutralization. Appl Microbiol. 1971 Jan;21(1):79–83. doi: 10.1128/am.21.1.79-83.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill G. S., Jr, Pederson C. E., Jr, Stookey J. L. A comparison of the tissue lesions produced in adult hamsters by two strains of avirulent Venezuelan equine encephalomyelitis virus. Am J Pathol. 1973 Jul;72(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- GLEISER C. A., GOCHENOUR W. S., Jr, BERGE T. O., TIGERTT W. D. The comparative pathology of experimental Venezuelan equine encephalomyelitis infection in different animal hosts. J Infect Dis. 1962 Jan-Feb;110:80–97. doi: 10.1093/infdis/110.1.80. [DOI] [PubMed] [Google Scholar]
- Gorelkin L., Jahrling P. B. Pancreatic involvement by Venezuelan equine encephalomyelitis virus in the hamster. Am J Pathol. 1974 May;75(2):349–362. [PMC free article] [PubMed] [Google Scholar]
- Hrusková J., Rychterová V., Kliment V. The influence of infection with Venezuelan equine encephalomyelitis virus on antibody response against sheep erythrocytes. I. Experiments on mice. Acta Virol. 1972 Mar;16(2):115–124. [PubMed] [Google Scholar]
- Hrusková J., Rychterová V., Kliment V. The influence of infection with Venezuelan equine encephalomyelitis virus on antibody response against sheep erythrocytes. II. Experiments on guinea pigs. Acta Virol. 1972 Mar;16(2):125–132. [PubMed] [Google Scholar]
- Jahrling P. B., Scherer F. Histopathology and distribution of viral antigens in hamsters infected with virulent and benign Venezuelan encephalitis viruses. Am J Pathol. 1973 Jul;72(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- KISSLING R. E., CHAMBERLAIN R. W., NELSON D. B., STAMM D. D. Venezuelan equine encephalomyelitis in horses. Am J Hyg. 1956 May;63(3):274–287. doi: 10.1093/oxfordjournals.aje.a119811. [DOI] [PubMed] [Google Scholar]
- Kundin W. D. Pathogenesis of Venezuelan equine encephalomyelitis virus. II. Infection in young adult mice. J Immunol. 1966 Jan;96(1):49–58. [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Monath T. P., Calisher C. H., Davis M., Bowen G. S., White J. Experimental studies of rhesus monkeys infected with epizootic and enzootic subtypes of Venezuelan equine encephalitis virus. J Infect Dis. 1974 Feb;129(2):194–200. doi: 10.1093/infdis/129.2.194. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Taylor W. P., Mims C. A., Marshall I. D. Pathogenesis of Ross River virus infection in mice. II. Muscle, heart, and brown fat lesions. J Infect Dis. 1973 Feb;127(2):129–138. doi: 10.1093/infdis/127.2.129. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Whitfield S. G. Eastern equine encephalitis virus infection: electron microscopic studies of mouse central nervous system. Exp Mol Pathol. 1970 Oct;13(2):131–146. doi: 10.1016/0014-4800(70)90001-8. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Winn W. C., Jr, Walker D. H., Flemister M. R., Whitfield S. G. Early lymphoreticular viral tropism and antigen persistence. Tamiami virus infection in the cotton rat. Lab Invest. 1976 Feb;34(2):125–140. [PubMed] [Google Scholar]
- TASKER J. B., MIESSE M. L., BERGE T. O. Studies on the virus of Venezuelan equine encephalomyelitis. III. Distribution in tissues of experimentally infected mice. Am J Trop Med Hyg. 1962 Nov;11:844–850. doi: 10.4269/ajtmh.1962.11.844. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VICTOR J., SMITH D. G., POLLACK A. D. The comparative pathology of Venezuelan equine encephalomyelitis. J Infect Dis. 1956 Jan-Feb;98(1):55–66. doi: 10.1093/infdis/98.1.55. [DOI] [PubMed] [Google Scholar]