Abstract
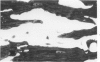
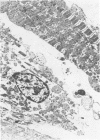
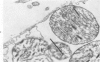
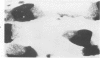
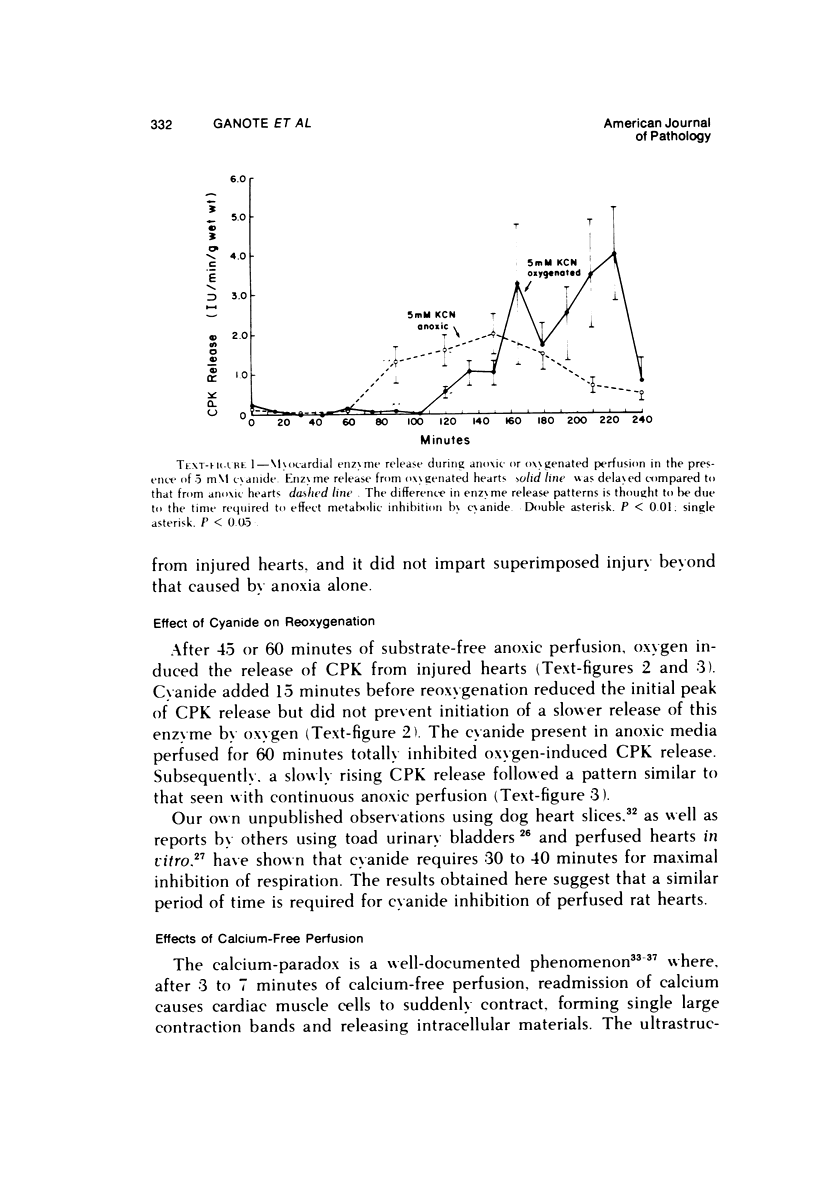
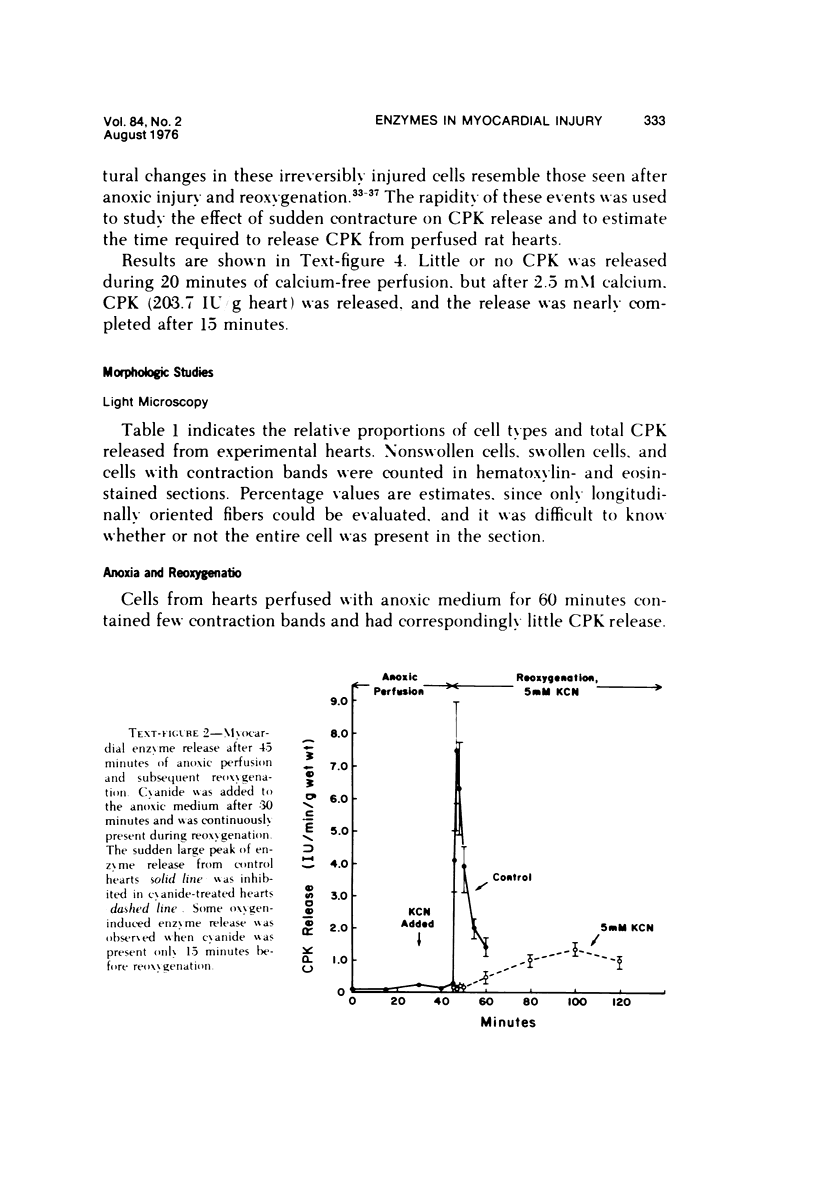
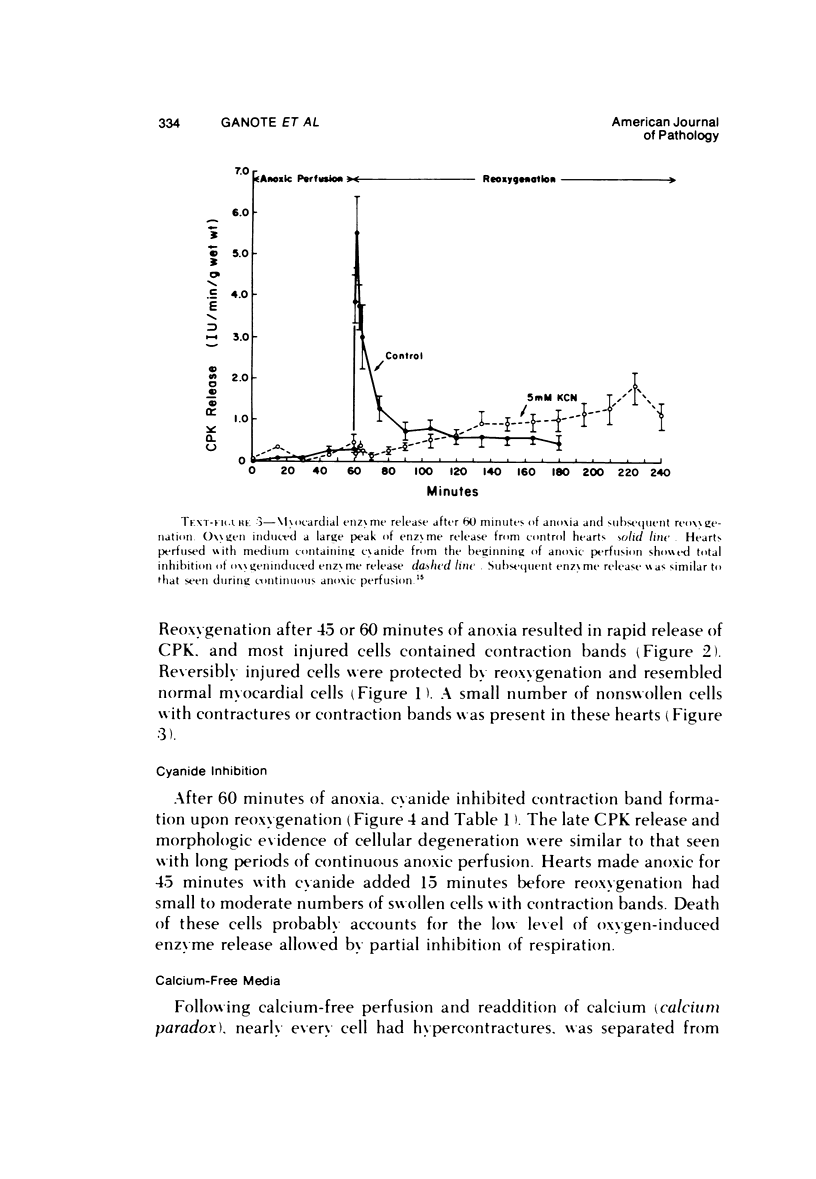
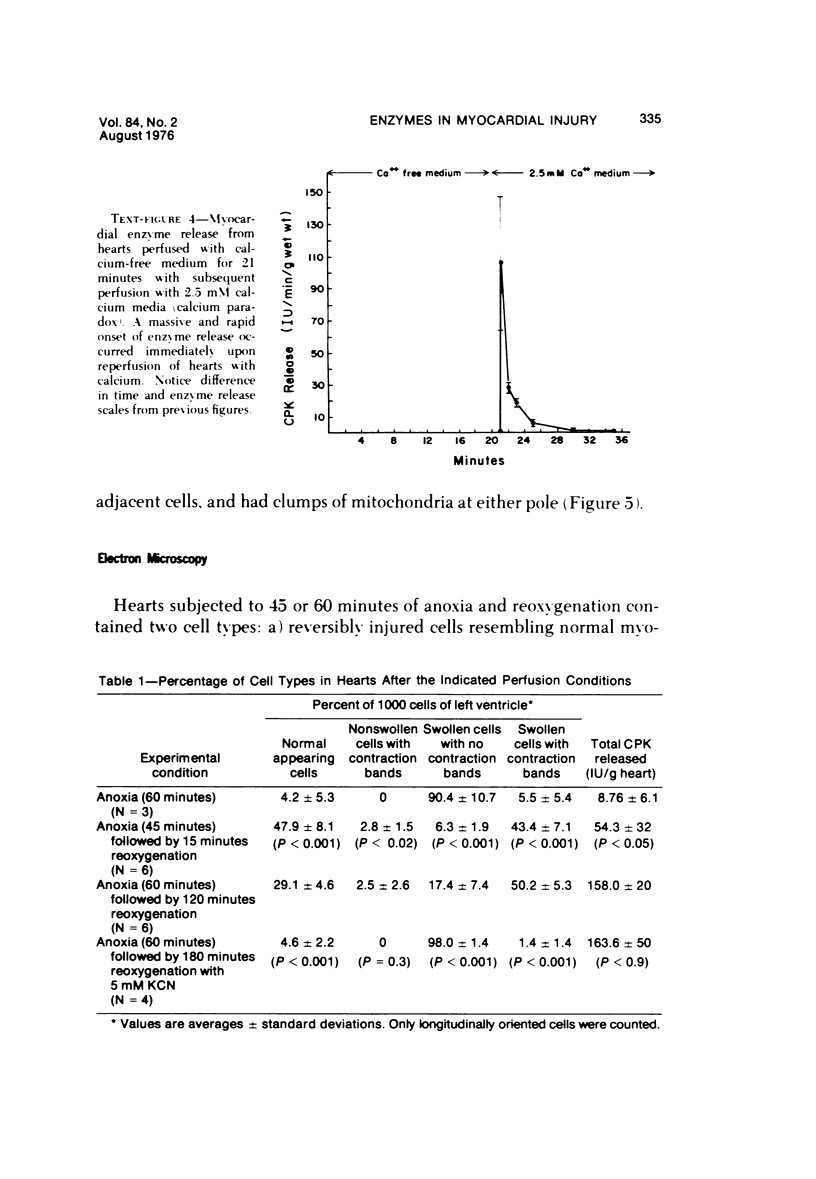
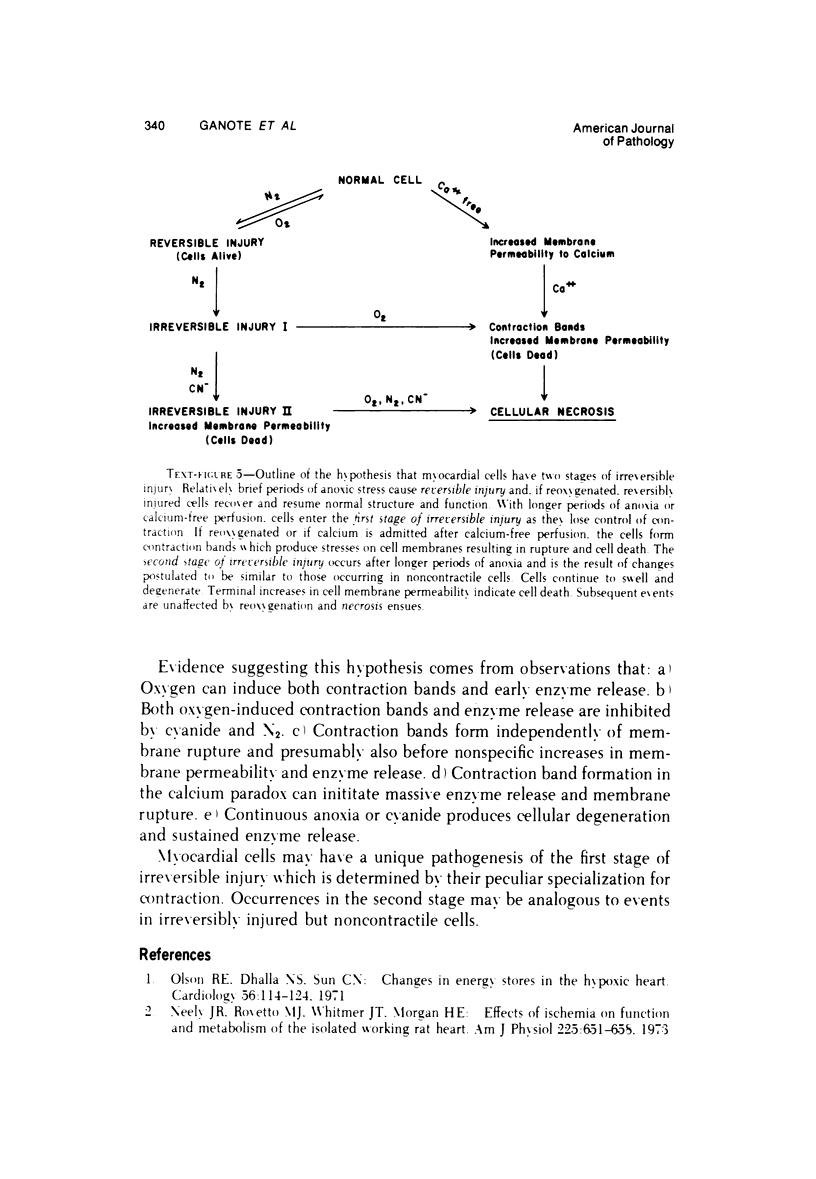
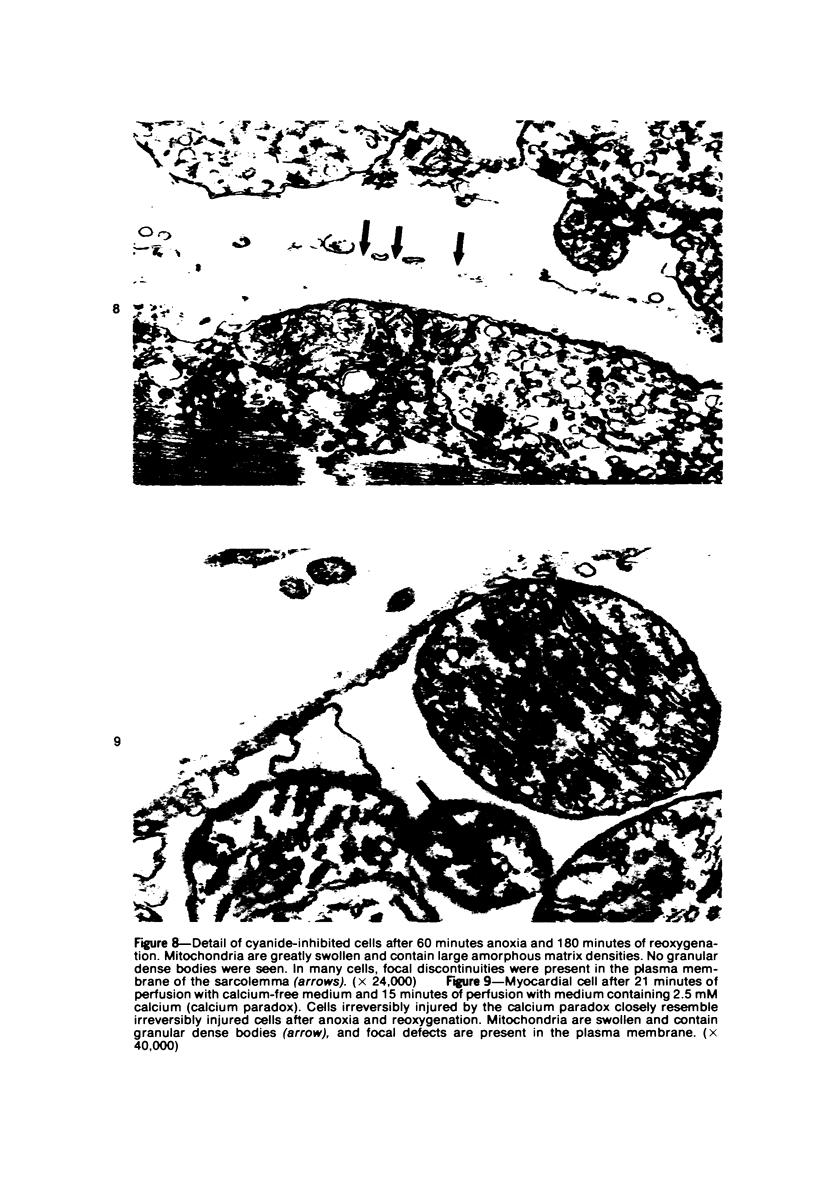
The effects of 5 mM potassium cyanide (KCN) on creatine phosphokinase (CPK) release and cellular morphology were studied. Rat hearts were perfused with substrate-deficient media gassed with O2 or N2 (O2 medium, N2 medium) at 37 C, and effluent was collected for creatine phosphokinase analysis. Tissue fixation was with glutaraldehyde for light and electron microscopy. Experiments included the following: a) continuous perfusion with O2- or N2-medium in the presence of KCN; b) 45 or 60 minutes of perfusion with N2-medium followed by O2-medium for 15 or 180 minutes, respectively; c) 45 minutes of perfusion with N2-medium with KCN added 15 minutes before reoxygenation with O2-medium plus KCN; (4) 60 minutes of N2-medium plus KCN followed by O2-medium plus KCN for 180 minutes; d) as a control for irreversible injury, 21 minutes of perfusion with calcium-free O2-medium followed by 2.5 mM calcium-O2-medium ("calcium paradox"). The following results were seen: a) Initial CPK release occurred about 30 minutes later from hearts perfused with O2-medium plus KCN than from hearts perfused with N2-medium plus KCN. b) Upon reoxygenation after either 45 or 60 minutes of anoxia, hearts had a sudden peak of oxygen-induced CPK release. Most irreversibly injured cells were massively swollen and had sarcolemmal defects and contraction bands. Reversibly injured cells in the same hearts resembled normal myocardium. A previously unrecognized third population of cells is described. These cells were characterized by contraction bands but were not swollen, had intact sarcolemma, and contained both normal and damaged mitochondria with intramatrical calcium accumulation granules. It could not be determined if these cells were reversibly injured or in an early stage of irreversible injury. c) KCN added 15 minutes before reoxygenation of hearts after 45 minutes of anoxia inhibited the sudden peak of oxygen-induced CPK release but not a slow sustained release. Small to moderate numbers of cells in these hearts contained contraction bands. d) After 60 minutes, KCN completely inhibited both oxygen-induced CPK release and contraction band formation. e) Addition of calcium to calcium-free hearts caused both massive CPK release and contraction band formation. It is concluded that: the beginning of CPK release from oxygenated KCN-inhibited hearts requires about 30 minutes longer than from anoxic hearts; KCN can inhibit both oxygen-induced CPK release and contraction bands in irreversibly injured rat myocardial cells; sudden contracture of myocardial cells as occurs in the calcium paradox can result in massive CPK release; contraction bands occur in nonswollen cells, hence contraction bands can occur independently of massive cell swelling or membrane rupture. It is postulated that there may be two stages of irreversible myocardial injury; a) loss of control of contraction and b) progressive loss of mitochondrial and membrane integrity.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinley F. J., Jr Calcium and magnesium transport in single cells. Fed Proc. 1973 Jul;32(7):1735–1739. [PubMed] [Google Scholar]
- Carafoli E. Mitochondria, Ca2+ transport and the regulation of heart contraction and metabolism. J Mol Cell Cardiol. 1975 Feb;7(2):83–87. doi: 10.1016/0022-2828(75)90010-3. [DOI] [PubMed] [Google Scholar]
- Case R. B., Nasser M. G., Crampton R. S. Biochemical aspects of early myocardial ischemia. Am J Cardiol. 1969 Dec;24(6):766–775. doi: 10.1016/0002-9149(69)90465-2. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., NAEGLE S., BING R. J. Effect of ischemia and reoxygenation on glycolytic reactions and adenosine-triphosphate in heart muscle. Circ Res. 1960 Sep;8:965–971. doi: 10.1161/01.res.8.5.965. [DOI] [PubMed] [Google Scholar]
- Fahimi H. D., Cotran R. S. Permeability studies in heat-induced injury of skeletal muscle using lanthanum as fine structural tracer. Am J Pathol. 1971 Jan;62(1):143–157. [PMC free article] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Ginn F. L., Shelburne J. D., Trump B. F. Disorders of cell volume regulation. I. Effects of inhibition of plasma membrane adenosine triphosphatase with ouabain. Am J Pathol. 1968 Dec;53(6):1041–1071. [PMC free article] [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R., Caplan A. I. Ion-induced ultrastructural transformations in isolated mitochondria. The energized uptake of calcium. J Cell Biol. 1969 Jul;42(1):221–234. doi: 10.1083/jcb.42.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G., Galante Y., Stiggall D. L. Mitochondrial ATP-Pi exchange complex and the site of uncoupling of oxidative phosphorylation. Fed Proc. 1975 Jul;34(8):1699–1706. [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Chain E. B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol. 1973 Aug;5(4):395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Nayler W. G., Slade A., Border D. Ultrastructural damage associated with reoxygenation of the anoxic myocardium. J Mol Cell Cardiol. 1975 May;7(5):315–324. doi: 10.1016/0022-2828(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Canessa-Fischer M. Sodium transport inhibition by selective mitochondrial inhibitors in the urinary bladder of the toad. J Cell Physiol. 1966 Oct;68(2):185–196. doi: 10.1002/jcp.1040680213. [DOI] [PubMed] [Google Scholar]
- IMAI S., RILEY A. L., BERNE R. M. EFFECT OF ISCHEMIA ON ADENINE NUCLEOTIDES IN CARDIAC AND SKELETAL MUSCLE. Circ Res. 1964 Nov;15:443–450. doi: 10.1161/01.res.15.5.443. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., BAUM J. H., HERDSON P. B. FINE STRUCTURAL CHANGES IN MYOCARDIAL ISCHEMIC INJURY. Arch Pathol. 1965 Feb;79:135–143. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Kloner R. A., Whalen D. A., Jr, Hamilton D. G. Explosive swelling of myocardial cells irreversibly injured by transient ischemia. Recent Adv Stud Cardiac Struct Metab. 1975;6:405–413. [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E., Reimer K. A. Ischemic tissue injury. Am J Pathol. 1975 Oct;81(1):179–198. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Kent S. P. Diffusion of plasma proteins into cells: a manifestation of cell injury in human myocardial ischemia. Am J Pathol. 1967 Apr;50(4):623–637. [PMC free article] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Laiho K. U., Trump B. F. Relationship of ionic, water, and cell volume changes in cellular injury of Ehrlich ascites tumor cells. Lab Invest. 1974 Sep;31(3):207–215. [PubMed] [Google Scholar]
- Legato M. J., Spiro D., Langer G. A. Ultrastructural alterations produced in mammalian myocardium by variation in perfusate ionic composition. J Cell Biol. 1968 Apr;37(1):1–12. doi: 10.1083/jcb.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legato M. J. The correlation of ultrastructure and function in the mammalian myocardial cell. Prog Cardiovasc Dis. 1969 Mar;11(5):391–409. doi: 10.1016/0033-0620(69)90028-0. [DOI] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G. Regulation of myocardial function--a subcellular phenomenon. J Mol Cell Cardiol. 1973 Jun;5(3):213–219. doi: 10.1016/0022-2828(73)90062-x. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R. E., Dhalla N. S., Sun C. N. Changes in energy stores in the hypoxic heart. Cardiology. 1971;56(1):114–124. doi: 10.1159/000169351. [DOI] [PubMed] [Google Scholar]
- Penttila A., Trump B. F. Extracellular acidosis protects Ehrlich ascites tumor cells and rat renal cortex against anoxic injury. Science. 1974 Jul 19;185(4147):277–278. doi: 10.1126/science.185.4147.277. [DOI] [PubMed] [Google Scholar]
- Ricciutti M. A. Lysosomes and myocardial cellular injury. Am J Cardiol. 1972 Oct;30(5):498–502. doi: 10.1016/0002-9149(72)90040-9. [DOI] [PubMed] [Google Scholar]
- Sahaphong S., Trump B. F. Studies of cellular injury in isolated kidney tubules of the flounder. V. Effects of inhibiting sulfhydryl groups of plasma membrane with the organic mercurials PCMB (parachloromercuribenzoate) and PCMB (parachloromercuribenzenesulfonate). Am J Pathol. 1971 May;63(2):277–298. [PMC free article] [PubMed] [Google Scholar]
- Schraer R., Elder J. A., Schraer H. Aspects of mitochondrial function in calcium movement and calcification. Fed Proc. 1973 Sep;32(9):1938–1943. [PubMed] [Google Scholar]
- Sommers H. M., Wessel H. U., Jennings R. B. Myocardial temperature changes in acute experimental infarction. Lab Invest. 1966 Dec;15(12):1982–1993. [PubMed] [Google Scholar]
- Trump B. F., Bulger R. E. Studies of cellular injury in isolated flounder tubules. IV. Electron microscopic observations of changes during the phase of altered homeostasis in tubules treated with cyanide. Lab Invest. 1968 Jun;18(6):731–739. [PubMed] [Google Scholar]
- Trump B. F., Ginn F. L. Studies of cellular injury in isolated flounder tubules. II. Cellular swelling in high potassium media. Lab Invest. 1968 Apr;18(4):341–351. [PubMed] [Google Scholar]
- Weissler A. M., Kruger F. A., Baba N., Scarpelli D. G., Leighton R. F., Gallimore J. K. Role of anaerobic metabolism in the preservation of functional capacity and structure of anoxic myocardium. J Clin Invest. 1968 Feb;47(2):403–416. doi: 10.1172/JCI105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]