Abstract
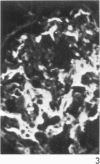
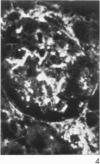
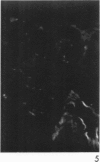
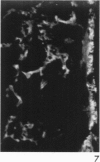
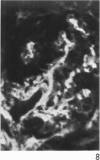
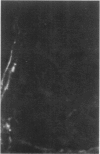
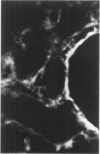
Antisera to human uterine actomyosin were prepared in rabbits and conjugated with fluorescein (F-AUAM). When F-AUAM was applied to frozen sections of normal human kidney which were then examined by ultraviolet light microscopy, it was observed that vascular smooth muscle, endothelium of arteries, veins, and peritubular capillaries and glomerular mesangial cells were immunofluorescent. Neither glomerular endothelium nor epithelial cells of Bowman's capsule or renal tubules were stained by F-AUAM. The specificity of antisera for actomyosin was confirmed by absorption and blocking studies, examination of a wide variety of tissues and immunodiffusion in agarose gel. It may be inferred from these data that mesangial cells are contractile. Contraction of the mesangium may play a significant role in regulating glomerular blood flow and in the reaction of the glomerulus to injury.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSCH K. G., GORDON G. B., MILLER L. FIBRILLAR STRUCTURES RESEMBLING LEIOMYOFIBRILS IN ENDOTHELIAL CELLS OF MAMMALIAN PULMONARY BLOOD VESSELS. Z Zellforsch Mikrosk Anat. 1964 Sep 3;63:759–766. doi: 10.1007/BF00336220. [DOI] [PubMed] [Google Scholar]
- BOYER C. C. The vascular pattern of the renal glomerulus as revealed by plastic reconstruction from serial sections. Anat Rec. 1956 Jul;125(3):433–441. doi: 10.1002/ar.1091250303. [DOI] [PubMed] [Google Scholar]
- BURKHOLDER P. M., LITTELL A. H., KLEIN P. G. Sectioning at room temperature of unfixed tissues, frozen in a gelatin matrix, for immunohistologic procedures. Stain Technol. 1961 Mar;36:89–91. doi: 10.3109/10520296109113250. [DOI] [PubMed] [Google Scholar]
- Becker C. G., Murphy G. E. Demonstration of contractile protein in endothelium and cells of the heart valves, endocardium, intima, arteriosclerotic plaques, and Aschoff bodies of rheumatic heart disease. Am J Pathol. 1969 Apr;55(1):1–37. [PMC free article] [PubMed] [Google Scholar]
- Bernik M. B. Contractile activity of human glomeruli in culture. Nephron. 1969;6(1):1–10. doi: 10.1159/000179708. [DOI] [PubMed] [Google Scholar]
- Finck H. Immunochemical studies on myosin. II. Cardiac myosin. Biochim Biophys Acta. 1965 Nov 15;111(1):221–230. doi: 10.1016/0304-4165(65)90488-5. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN G., SLIZYS I. S., CHASE M. W. Studies on fluorescent antibody staining. I. Non-specific fluorescence with fluorescein-coupled sheep anti-rabbit globulins. J Exp Med. 1961 Jul 1;114:89–110. doi: 10.1084/jem.114.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIBBS R. G., BURCH G. E., PHILLIPS J. H. The fine structure of the small blood vessels of normal human dermis and subcutis. Am Heart J. 1958 Nov;56(5):662–670. doi: 10.1016/0002-8703(58)90209-6. [DOI] [PubMed] [Google Scholar]
- HUHN D., STEINER J. W., MOVAT H. Z. [The fine structure of the mesangium in the kidney glomerulus of the dog and mouse]. Z Zellforsch Mikrosk Anat. 1962;56:213–230. [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. Relations of the centrolobular region of the glomerulus to the juxtaglomerular apparatus. J Ultrastruct Res. 1962 Jun;6:562–578. doi: 10.1016/s0022-5320(62)80010-0. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. The juxtaglomerular apparatus as studied electron microscopically. J Ultrastruct Res. 1962 Jun;6:547–561. doi: 10.1016/s0022-5320(62)80009-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEASE D. C., MOLINARI S. Electron microscopy of muscular arteries; pial vessels of43 the cat and monkey. J Ultrastruct Res. 1960 Jun;3:447–468. doi: 10.1016/s0022-5320(60)90022-8. [DOI] [PubMed] [Google Scholar]
- RHODIN J. A. Fine structure of vascular walls in mammals with special reference to smooth muscle component. Physiol Rev Suppl. 1962 Jul;5:48–87. [PubMed] [Google Scholar]
- Ross M. H., Reith E. J. Myoid elements in the mammalian nephron and their relationship to other specializations in the basal part of kidney tubule cells. Am J Anat. 1970 Dec;129(4):399–415. doi: 10.1002/aja.1001290403. [DOI] [PubMed] [Google Scholar]
- YAMADA E. The fine structure of the renal glomerulus of the mouse. J Biophys Biochem Cytol. 1955 Nov 25;1(6):551–566. doi: 10.1083/jcb.1.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]