Abstract
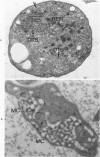
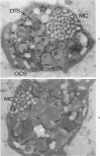
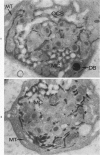
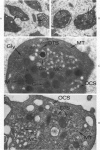
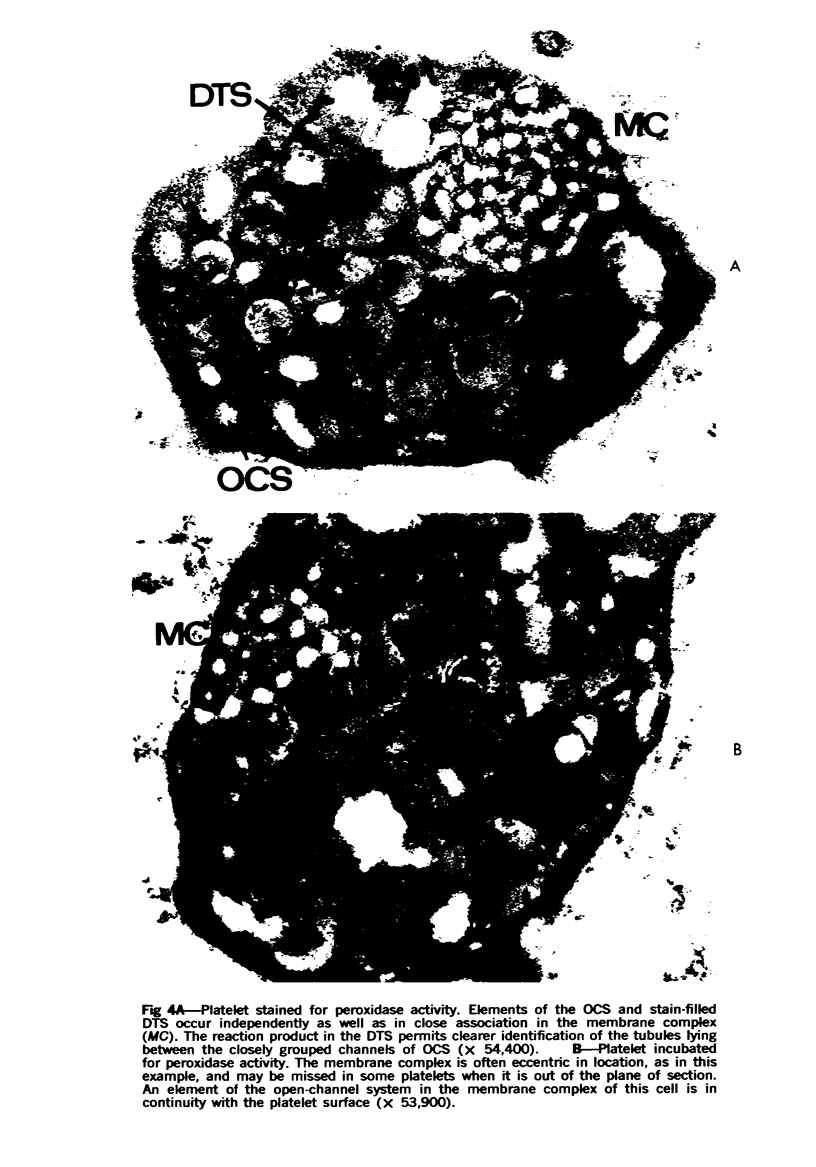
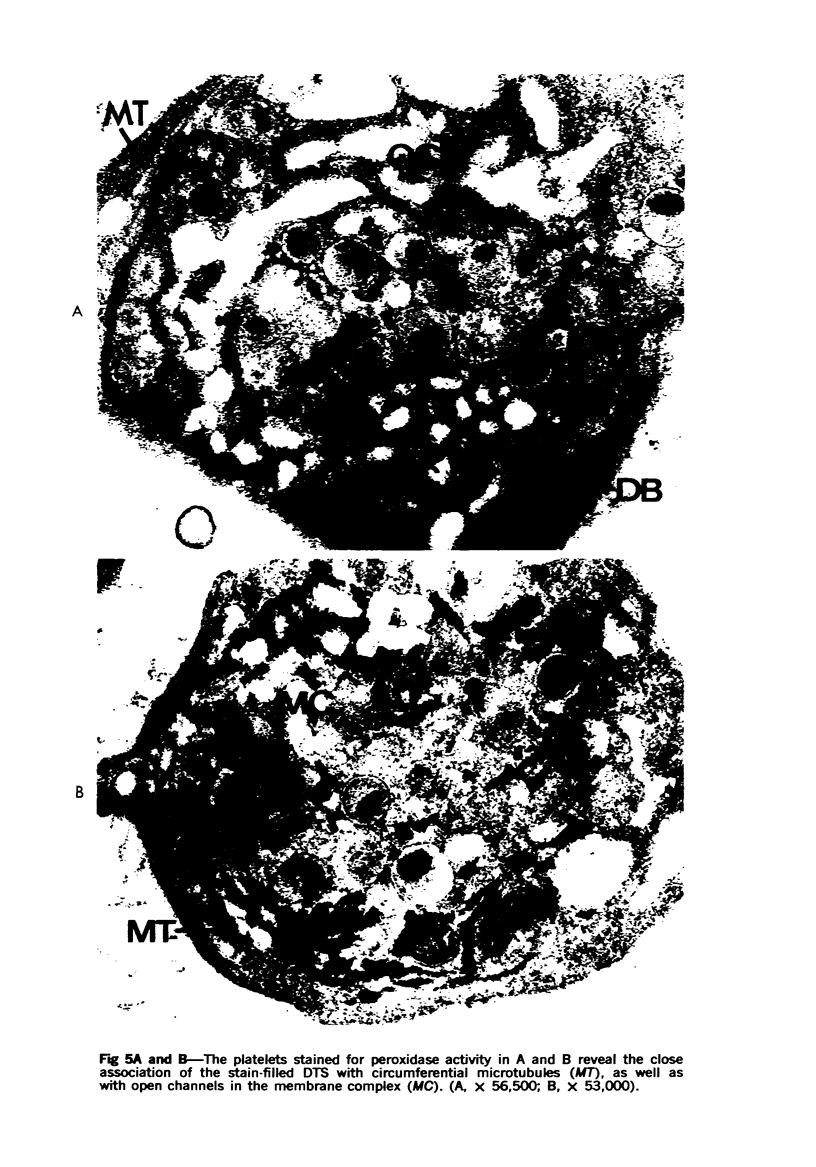
The existence of two separate groups of membrane-enclosed channels in platelets, the open canalicular system (OCS) and the dense tubular system (DTS), has been recognized for several years. The present study has employed ultrastructural observations on normal platelets, cells from a child with the gray platelet syndrome and cytochemical localization of peroxidase in platelets to define the origin of the DTS and point out a specialized membrane complex in platelets formed by interaction of channels from the OCS and DTS. The similarities of membrane complexes in platelets to the organization of membrane systems in muscle is pointed out, and their potential function as the sarcoplasmic reticulum of platelets is discussed.
Full text
PDF











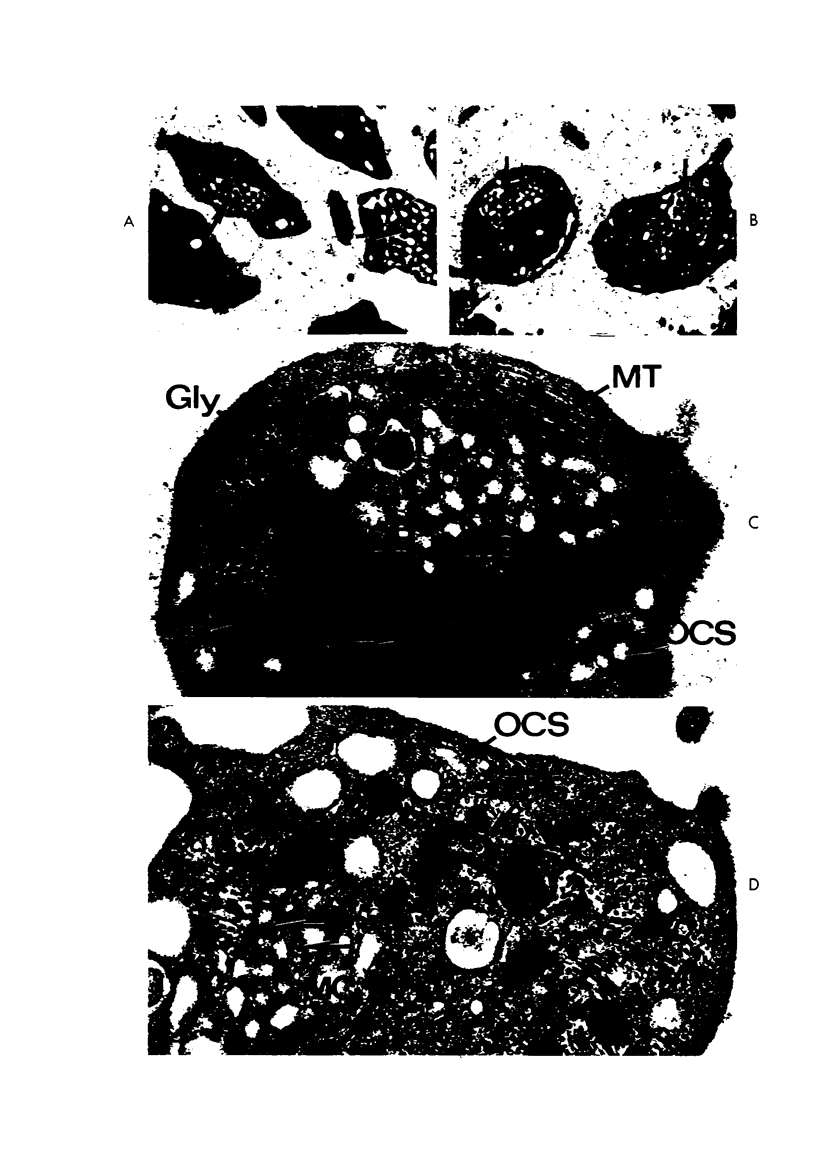

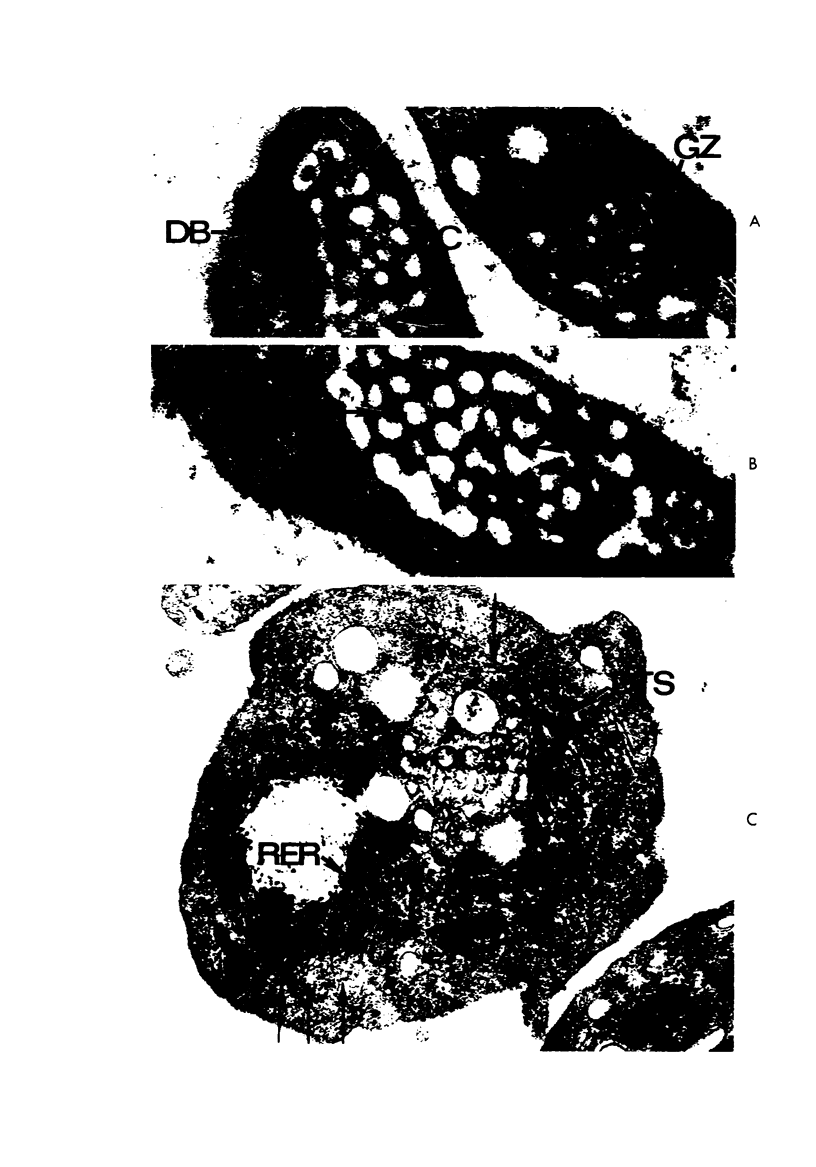
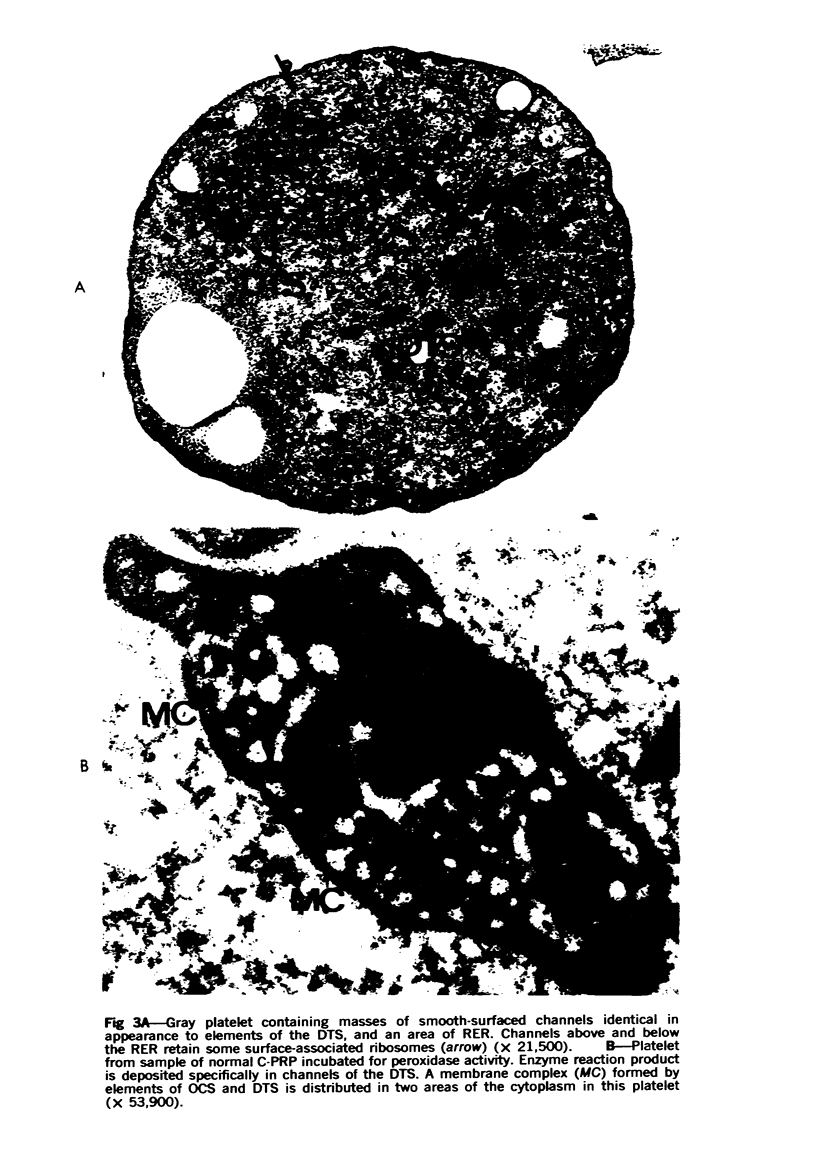
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J Ultrastruct Res. 1968 Sep;24(5):412–433. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- Behnke O. Further studies on microtubules. A marginal bundle in human and rat thrombocytes. J Ultrastruct Res. 1965 Dec;13(5):469–477. doi: 10.1016/s0022-5320(65)90009-2. [DOI] [PubMed] [Google Scholar]
- Essner E. Localization of endogenous peroxidase in rat exorbital lacrimal gland. J Histochem Cytochem. 1971 Apr;19(4):216–225. doi: 10.1177/19.4.216. [DOI] [PubMed] [Google Scholar]
- FIRKIN B. G., O'NEILL B. J., DUNSTAN B., OLDFIELD R. THE EFFECT OF INCUBATION AND STORAGE ON HUMAN PLATELET STRUCTURE AS STUDIED BY ELECTRON MICROSCOPY. Blood. 1965 Mar;25:345–355. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hagopian M., Spiro D. The sarcoplasmic reticulum and its association with the T system in an insect. J Cell Biol. 1967 Mar;32(3):535–545. doi: 10.1083/jcb.32.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. Metabolic and kinetic evidence suggestive of young and old platelets. Ser Haematol. 1971;4(1):75–97. [PubMed] [Google Scholar]
- Legg P. G., Wood R. L. New observations on microbodies. A cytochemical study on CPIB-treated rat liver. J Cell Biol. 1970 Apr;45(1):118–129. doi: 10.1083/jcb.45.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statland B. E., Heagan B. M., White J. G. Uptake of calcium by platelet relaxing factor. Nature. 1969 Aug 2;223(5205):521–522. doi: 10.1038/223521a0. [DOI] [PubMed] [Google Scholar]
- WHITE J. G., KRIVIT W., VERNIER R. L. THE PLATELET-FIBRIN RELATIONSHIP IN HUMAN BLOOD CLOTS: AN ULTRASTRUCTURAL STUDY UTILIZING FERRITIN-CONJUGATED ANTI-HUMAN FIBRINOGEN ANTIBODY. Blood. 1965 Feb;25:241–257. [PubMed] [Google Scholar]
- White J. G. Effects of colchicine and vinca alkaloids on human platelets. II. Changes in the dense tubular system and formation of an unusual inclusion in incubated cells. Am J Pathol. 1968 Sep;53(3):447–461. [PMC free article] [PubMed] [Google Scholar]
- White J. G. The submembrane filaments of blood platelets. Am J Pathol. 1969 Aug;56(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- White J. G. The transfer of thorium particles from plasma to platelets and platelet granules. Am J Pathol. 1968 Oct;53(4):567–575. [PMC free article] [PubMed] [Google Scholar]