Abstract
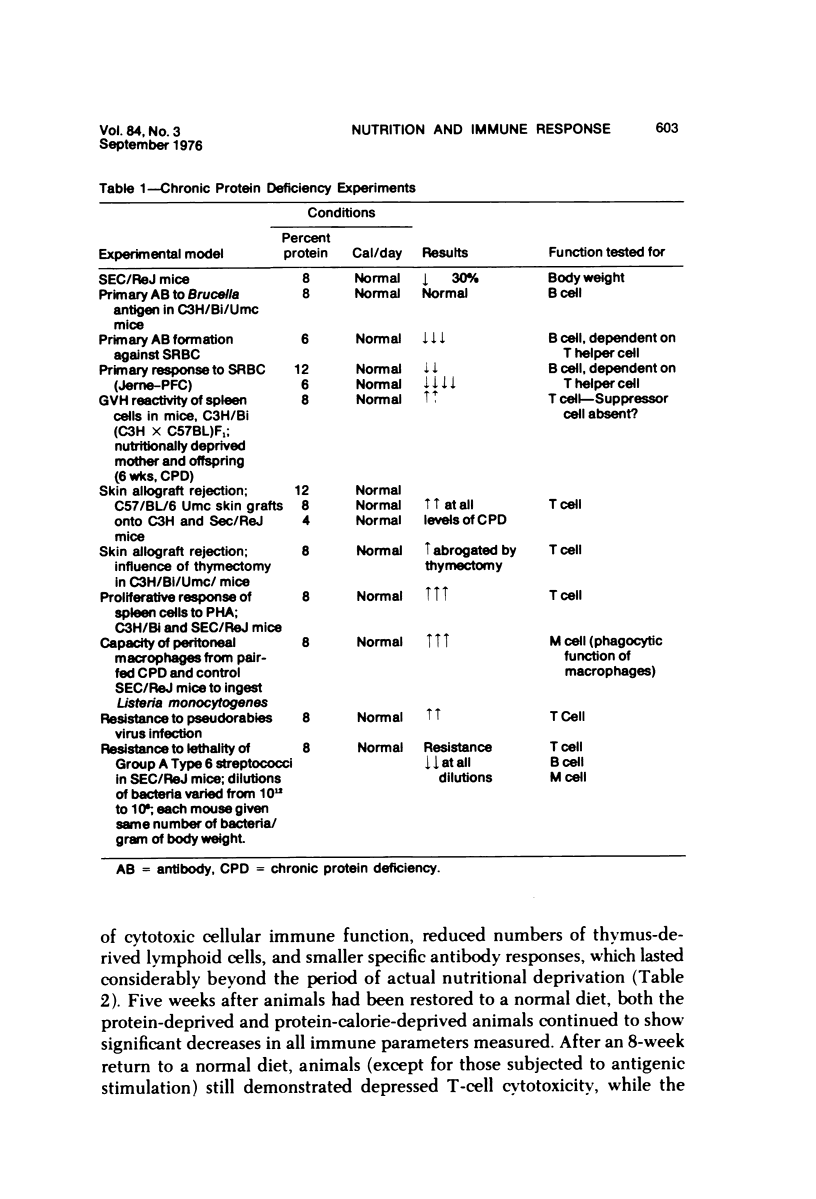
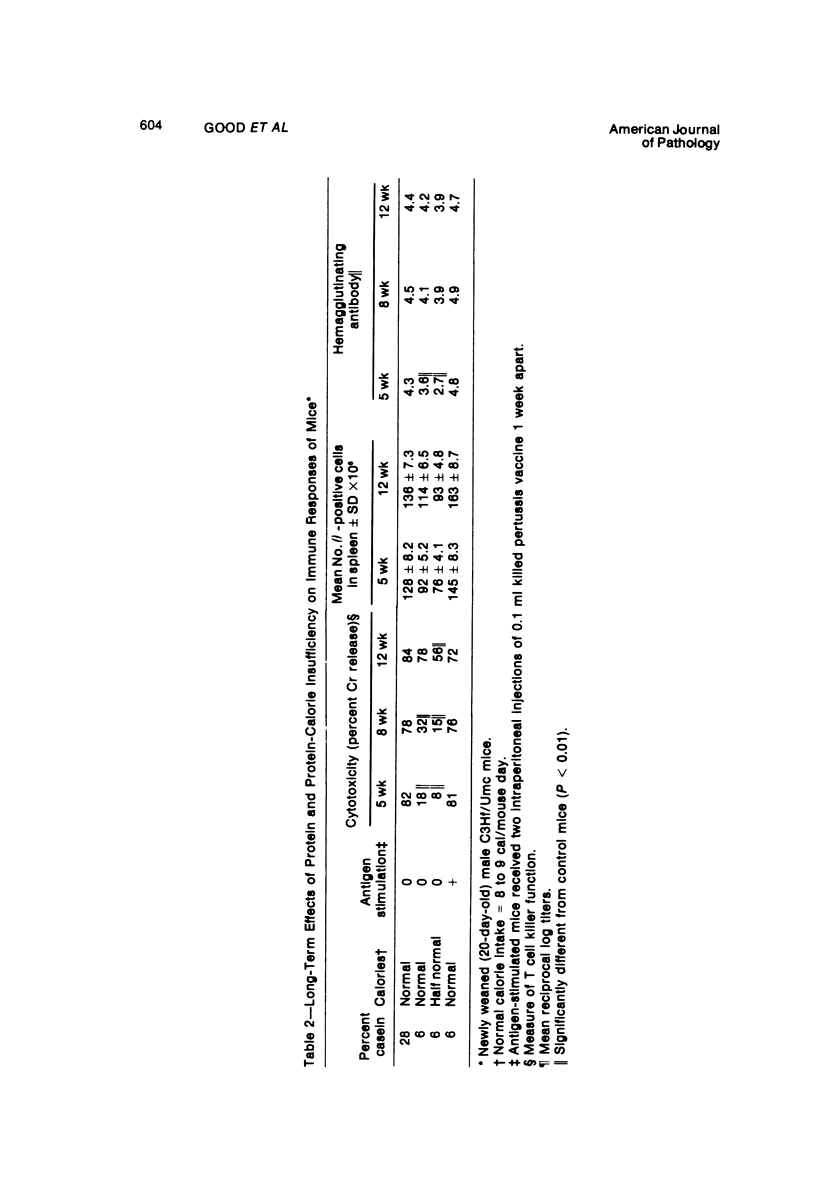
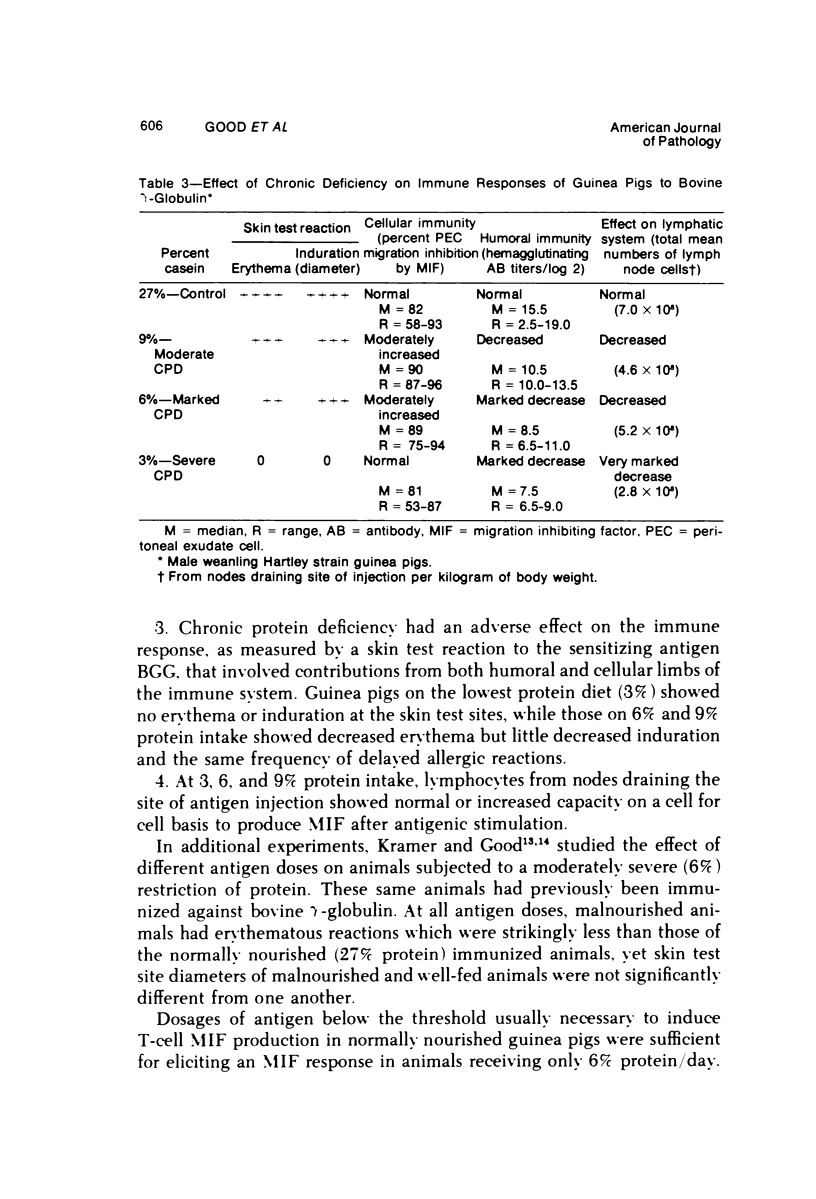
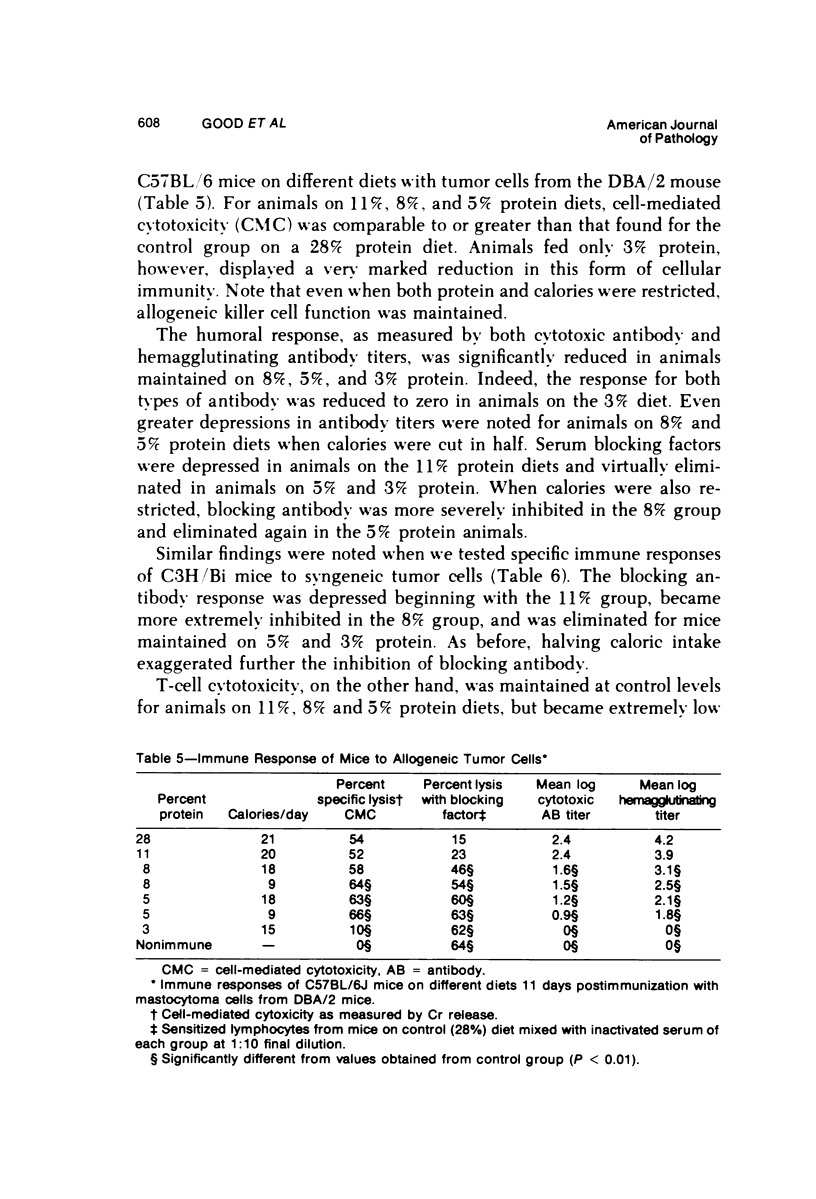
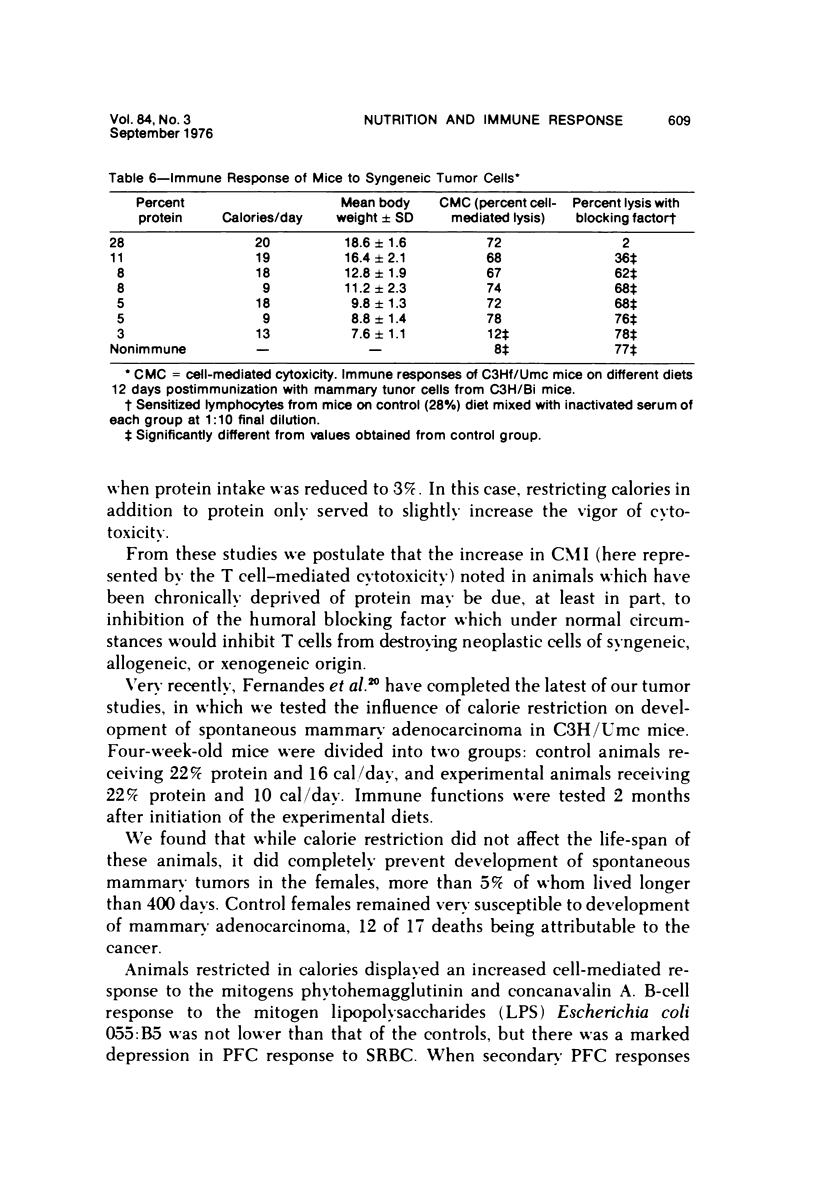
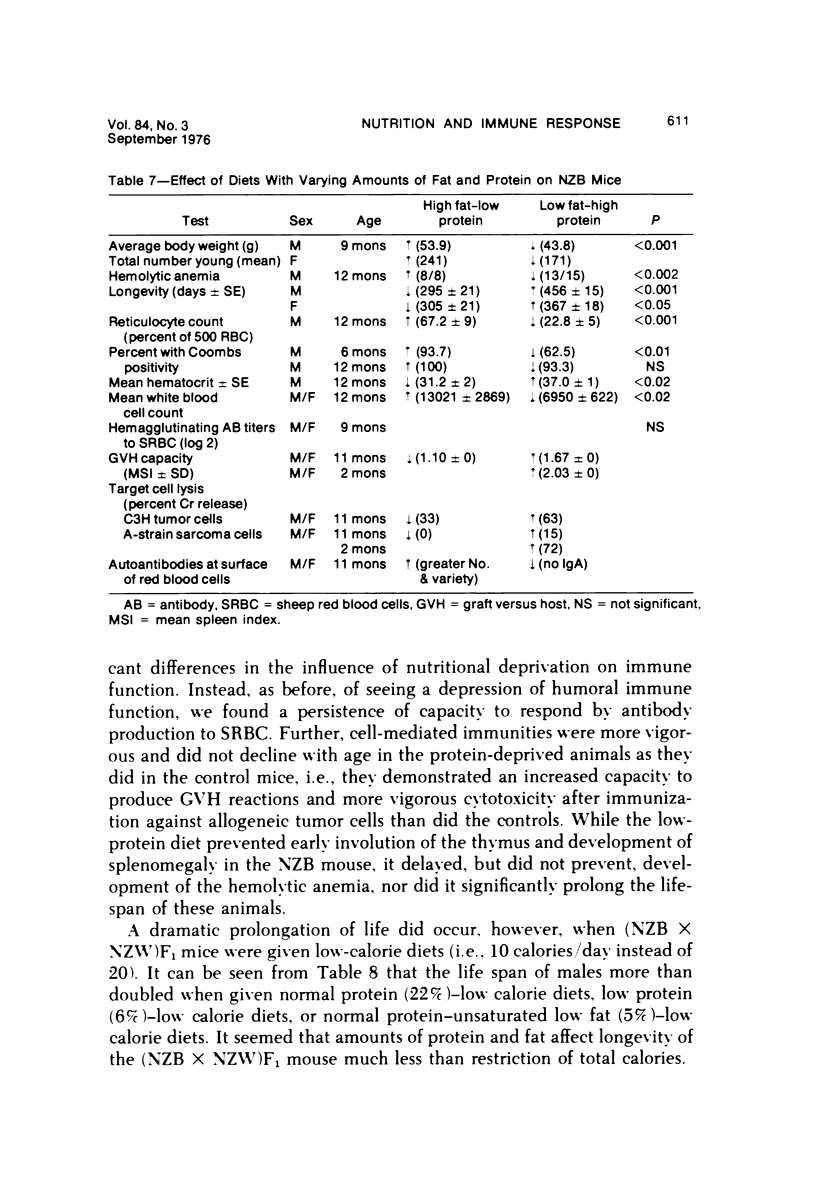
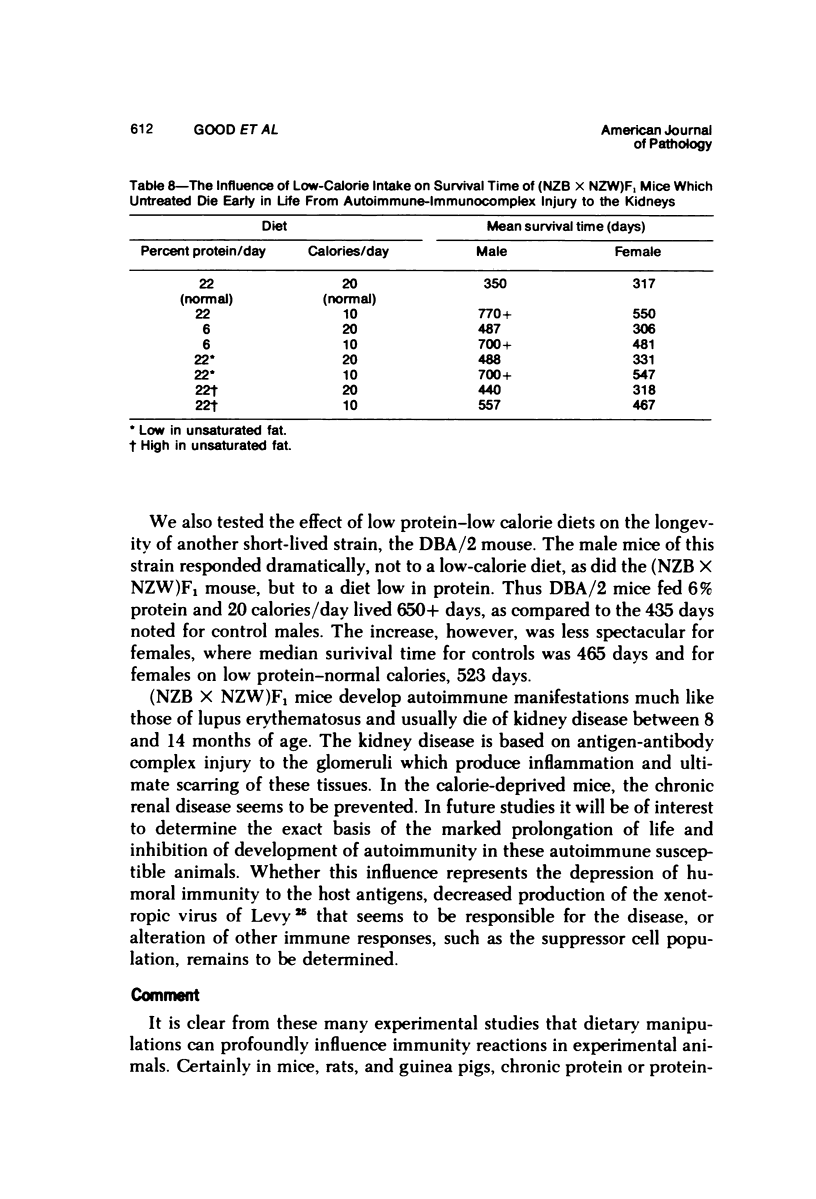
Several experiments conducted by our group over a period of 6 years have shown that nutritional stress, especially protein and/or calorie deprivation, leads to many, often dramatic, changes in the immune responses of mice, rats, and guinea pigs. Chronic protein deprivation (CPD) has been shown to create an enhancing effect on the cell-mediated immune responses of these animals. Humoral responses under CPD conditions were most often found to be depressed, but sometimes were unaffected, depending on the nature of the antigen employed. Chronic protein deprivation, consistent with the pattern just mentioned, improved tumor immunity by depressing production of B-cell blocking factors, and, in at least one instance, resistance to development of mammary adenocarcinoma in C3H mice was associated with evidence of increased numbers of T suppressor cells. Profound nutritional deficits (less than 5% protein per total daily food intake) depressed both cellular and humoral immunity. Early, though temporary, protein deprivation caused a long-term depression of both cellular and humoral immunity also, with the humoral component being the first to recover. Manipulation of protein and calories was found to have a profound effect on certain autoimmune conditions. Diets high in fat and low in protein favored reproduction but shortened the life of NZB mice, whereas diets high in protein and low in fat inhibited development of autoimmunity and prolonged life. Chronic moderate protein restriction permitted NZB mice to maintain their normally waning immunologic functions much longer than mice fed a normal protein intake. Further, the low-protein diet was associated with a delay in development of manifestations of autoimmunity. Decreasing dietary calories by a reduction of fats, carbohydrates, and proteins more than doubled the average life span of (NZB X NZW)F1 mice, a strain prone to early death from autoimmune disease. Histopathologic studies using immunofluorescent microscopy revealed that the development of the renal lesions caused by the deposition of antigen-antibody complexes, which is so characteristic of these mice, was markedly delayed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aref G. H., el-Din M. K., Hassan A. I., Araby I. I. Immunoglobulins in kwashiorkor. J Trop Med Hyg. 1970 Aug;73(8):186–191. [PubMed] [Google Scholar]
- Brown R. E., Katz M. Failure of antibody production to yellow fever vaccine in children with kwashiorkor. Trop Geogr Med. 1966 Jun;18(2):125–128. [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of protein restriction on immune functions in NZB mice. J Immunol. 1976 Mar;116(3):782–790. [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Jose D. G., Good R. A. Dietary influence on antinuclear antibodies and cell-mediated immunity in NZB mice. Int Arch Allergy Appl Immunol. 1973;44(6):770–782. doi: 10.1159/000230981. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Smith J., Good R. A. Dietary influence on breeding behavior, hemolytic anemia, and longevity in NZB mice. Proc Soc Exp Biol Med. 1972 Apr;139(4):1189–1196. doi: 10.3181/00379727-139-36327. [DOI] [PubMed] [Google Scholar]
- Harland P. S. Tuberculin reactions in malnourished children. Lancet. 1965 Oct 9;2(7415):719–721. doi: 10.1016/s0140-6736(65)90457-5. [DOI] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Absence of enhancing antibody in cell mediated immunity to tumour heterografts in protein deficient rats. Nature. 1971 Jun 4;231(5301):323–325. doi: 10.1038/231323b0. [DOI] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Quantitative effects of nutritional protein and calorie deficiency upon immune responses to tumors in mice. Cancer Res. 1973 Apr;33(4):807–812. [PubMed] [Google Scholar]
- Jose D. G., Stutman O., Good R. A. Long term effects on immune function of early nutritional deprivation. Nature. 1973 Jan 5;241(5384):57–58. doi: 10.1038/241057a0. [DOI] [PubMed] [Google Scholar]
- Jose D. G., Welch J. S., Doherty P. L. Humoral and cellular immune responses to streptococci, influenza and other antigens in Australian aboriginal school children. Aust Paediatr J. 1970 Dec;6(4):192–202. [PubMed] [Google Scholar]
- Levy J. A., Pincus T. Demonstration of biological activity of a murine leukemia virus of New Zealand black mice. Science. 1970 Oct 16;170(3955):326–327. doi: 10.1126/science.170.3955.326. [DOI] [PubMed] [Google Scholar]
- Mathews J. D., Mackay I. R., Whittingham S., Malcolm L. A. Protein supplementation and enhanced antibody-producing capacity in New Guinean school-children. Lancet. 1972 Sep 30;2(7779):675–677. doi: 10.1016/s0140-6736(72)92086-7. [DOI] [PubMed] [Google Scholar]
- Smythe P. M., Brereton-Stiles G. G., Grace H. J., Mafoyane A., Schonland M., Coovadia H. M., Loening W. E., Parent M. A., Vos G. H. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet. 1971 Oct 30;2(7731):939–943. doi: 10.1016/s0140-6736(71)90267-4. [DOI] [PubMed] [Google Scholar]


