Abstract
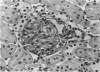
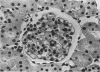
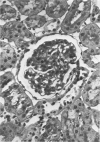
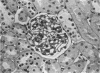
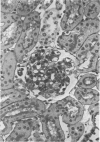
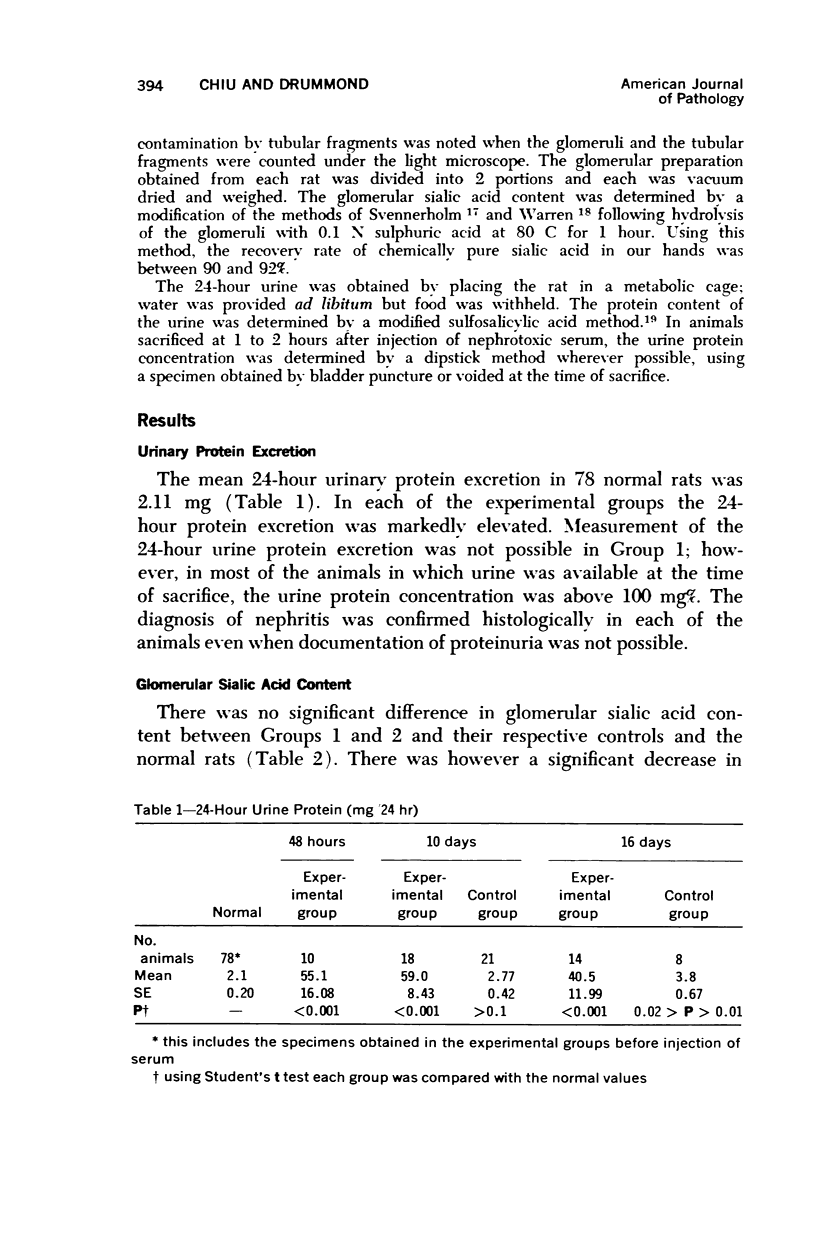
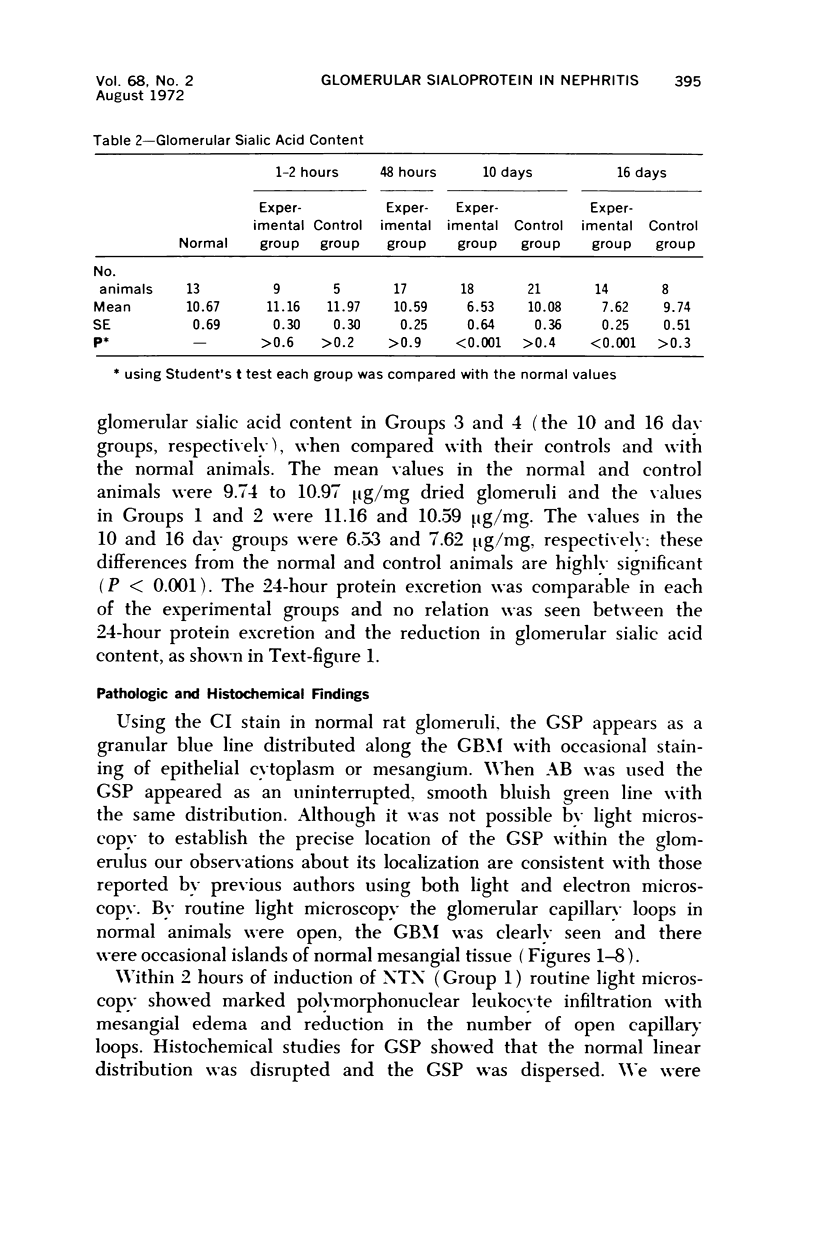
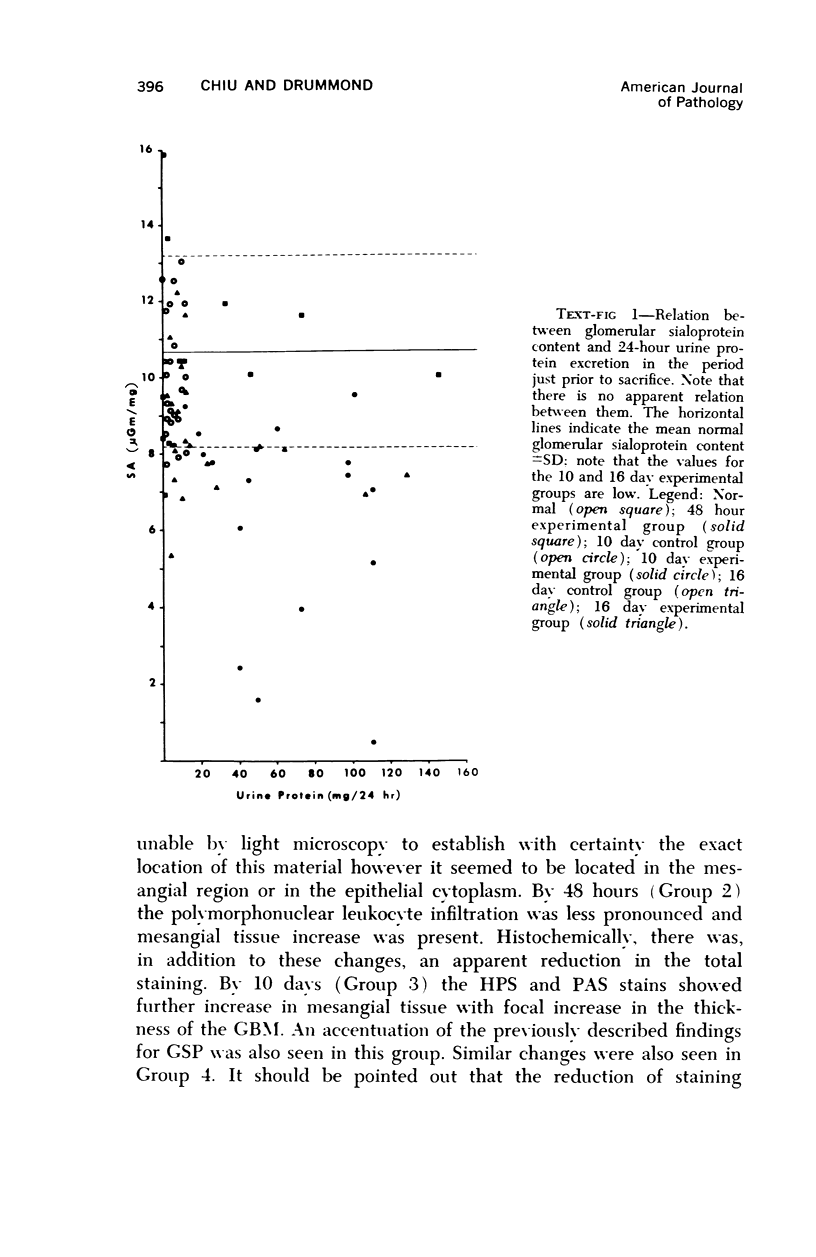
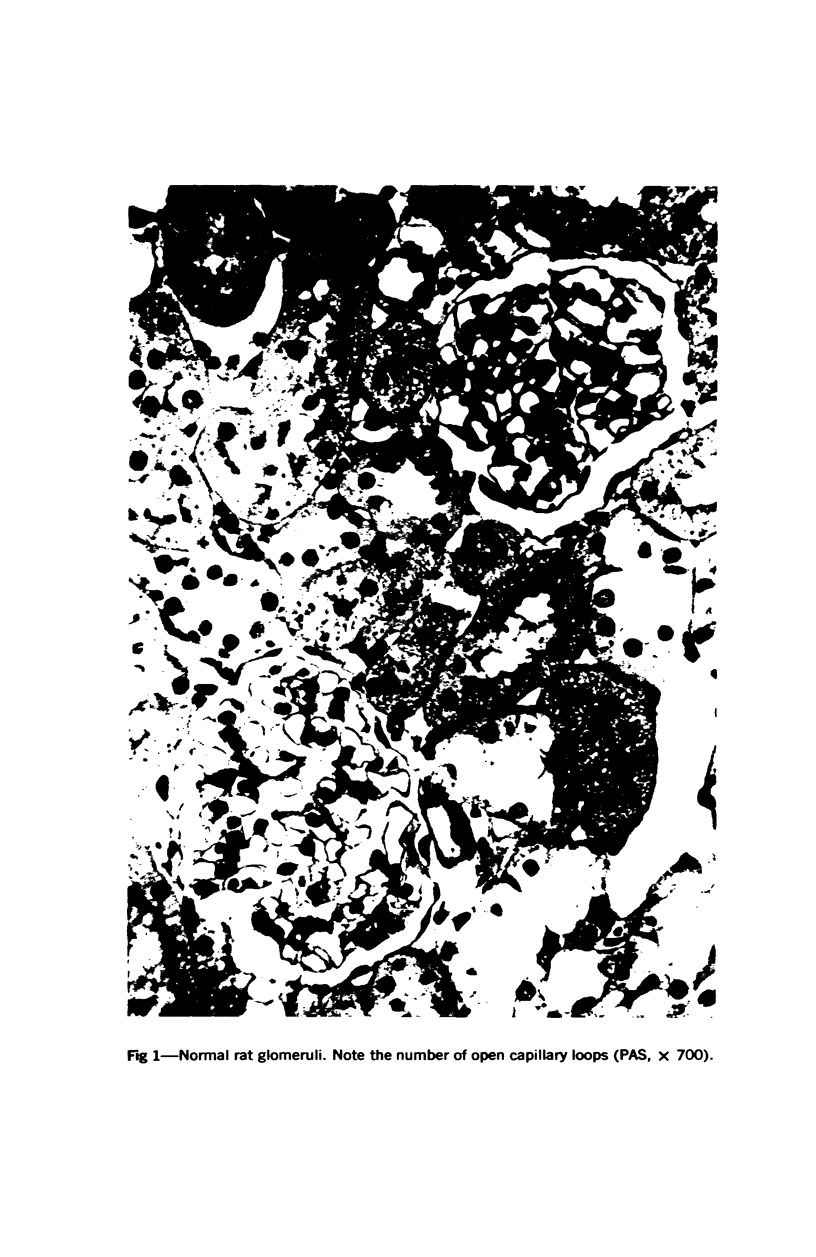
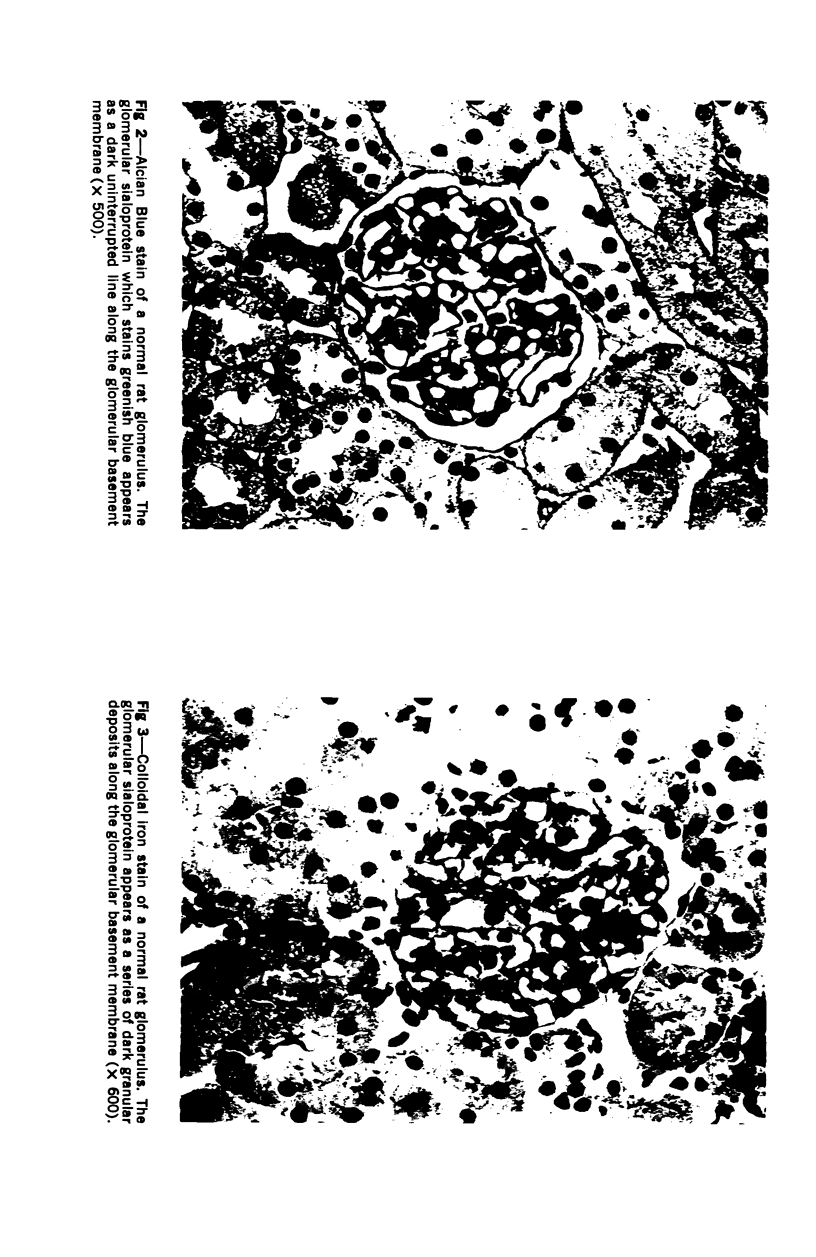
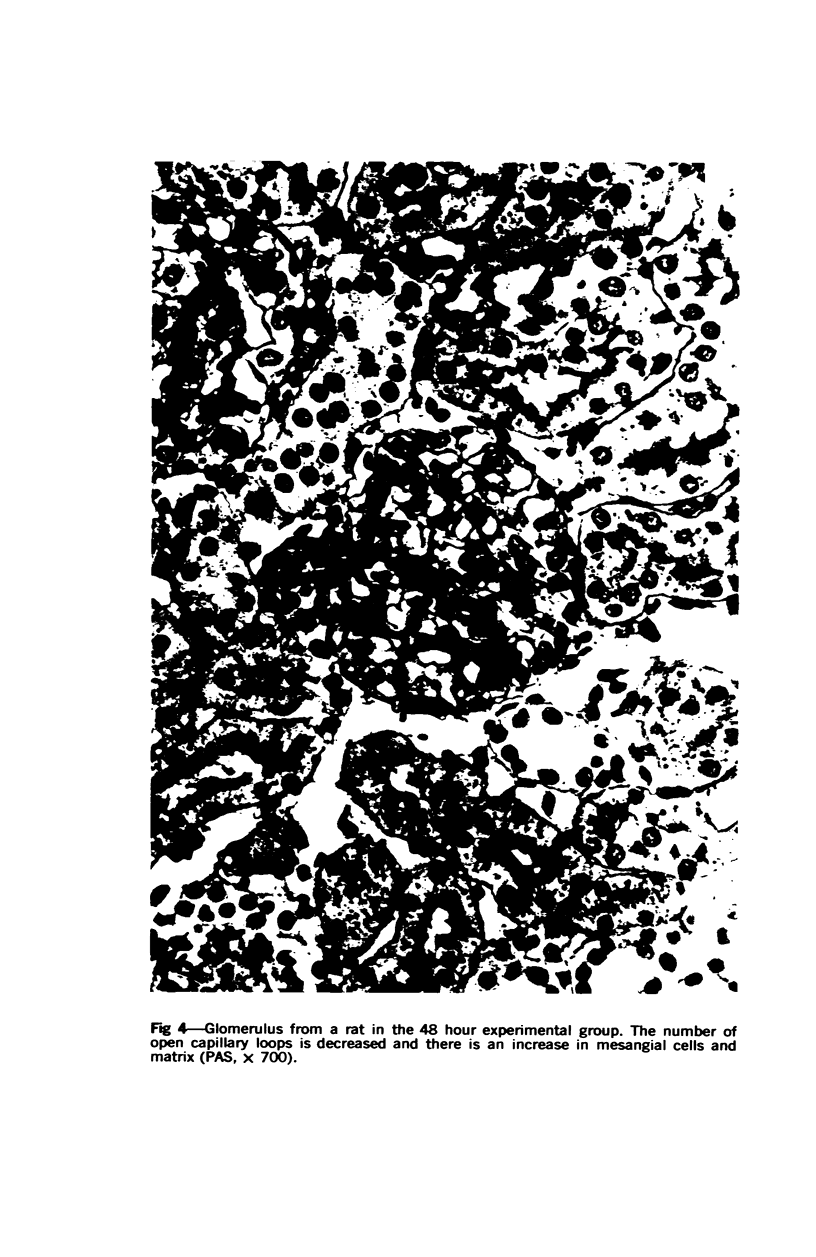
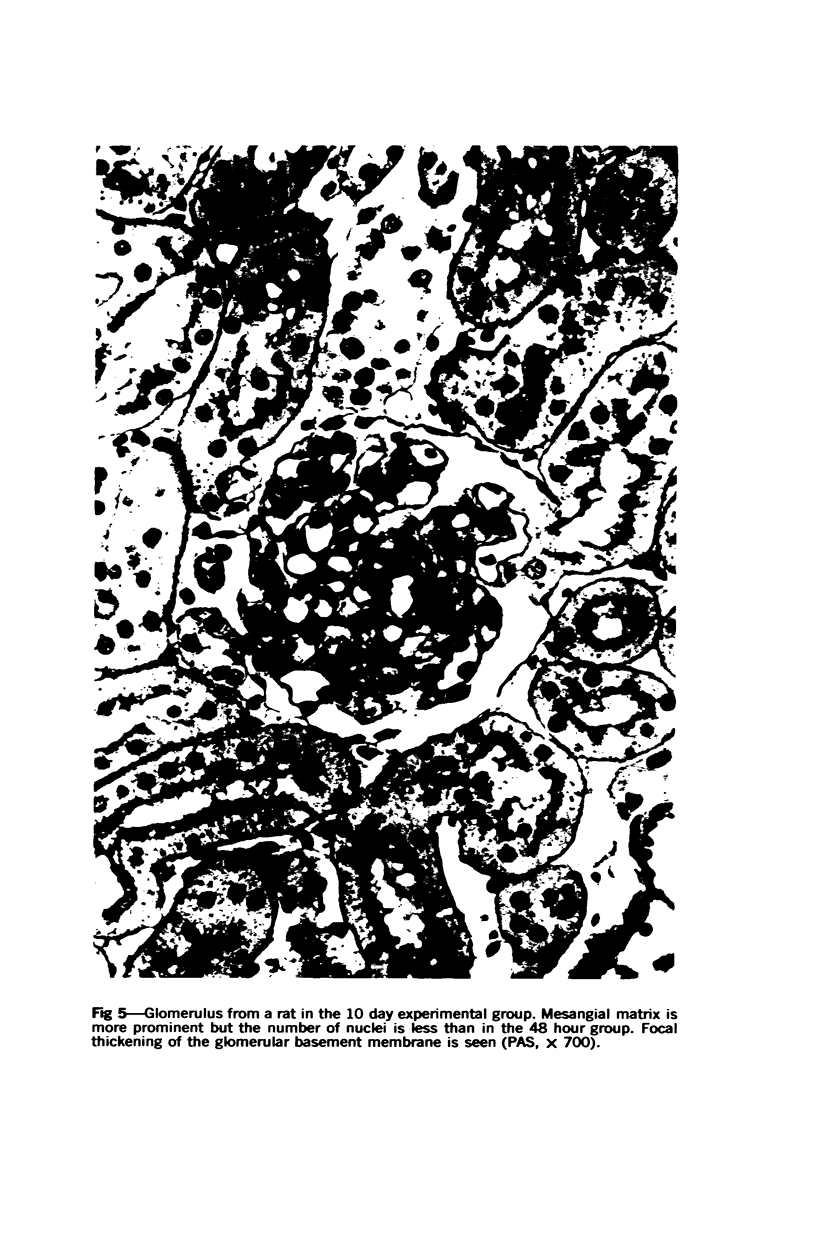
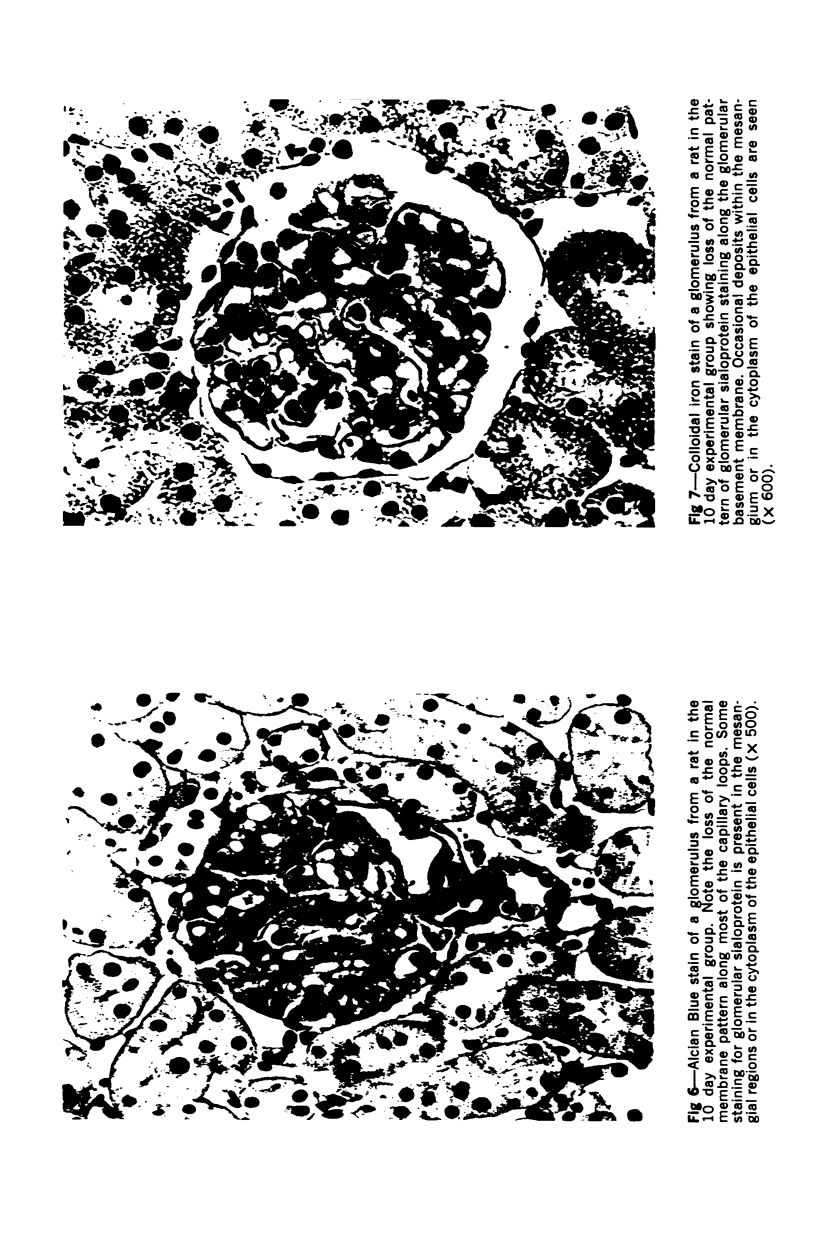
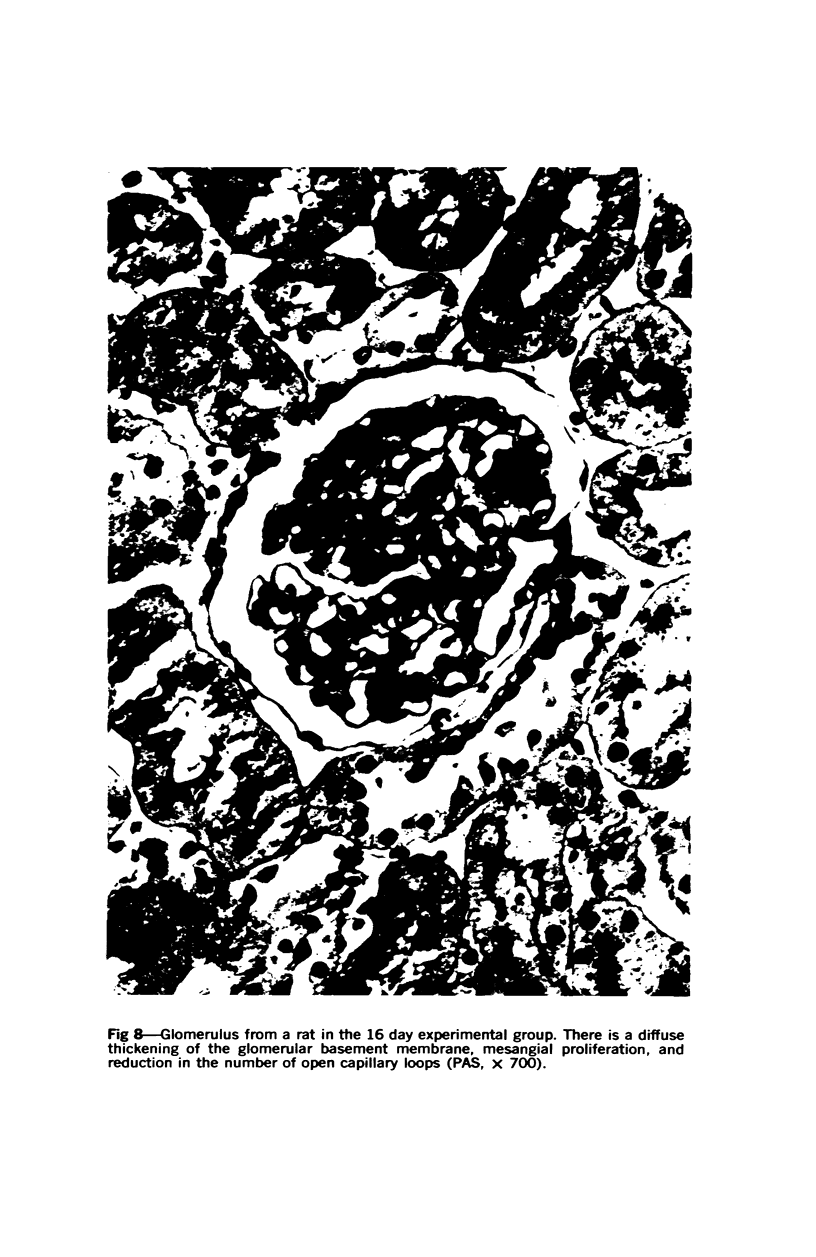
Glomerular sialoprotein (GSP) in nephrotoxic nephritis (NTN) rats was studied by chemical and histochemical methods at 1 to 2 hours, 48 hours, 10 days and 16 days after the induction of the disease by intravenous injection of rabbit antiserum to whole rat glomeruli. Histochemically, GSP was demonstrated by the CI and AB 8 GX stains. After the specimen was taken for histologic studies, the glomeruli from the kidneys of each rat were isolated by differential seiving and centrifugation. Quantitative determination of the glomerular sialic acid content from each rat was done using a combination of the chromatographic method of Svennerholm and the thiobarbituric acid assay of Warren. In normal rats the GSP is seen on the epithelial aspect of the glomerular basement membrane (GBM). Within 1 to 2 hours after induction of NTN, disruptuin of the normal membranous distribution of GSP and its dispersion into the mesangial space or the epithelial cytoplasm was noted. Reduction in the amount of staining was also observed after 48 hours. These changes increased in severity as the histologic lesions progressed. A quantitative decrease in glomerular sialic acid became apparent 10 days after the onset of NTN and was still observed at 16 days. Since GSP is situated at the filtration barrier site and is considered a major component of the glomerular antigenic structure, its changes in NTN suggest that it may play an important role in the pathogenesis of this disease and in the alteration of GBM permeability.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Graham R. C., Jr, Karnovsky M. J. Glomerular permeability. Ultrastructural cytochemical studies using peroxidases as protein tracers. J Exp Med. 1966 Dec 1;124(6):1123–1134. doi: 10.1084/jem.124.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL V. The protoplasmic basis of glomerular ultrafiltration. Am Heart J. 1957 Jul;54(1):1–9. doi: 10.1016/0002-8703(57)90073-x. [DOI] [PubMed] [Google Scholar]
- Ito S. Structure and function of the glycocalyx. Fed Proc. 1969 Jan-Feb;28(1):12–25. [PubMed] [Google Scholar]
- Jones D. B. Mucosubstances of the glomerulus. Lab Invest. 1969 Aug;21(2):119–125. [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohos S. C., Skoza L. Variations in the sialic acid concentration of glomerular basement membrane preparations obtained by ultrasonic treatment. J Cell Biol. 1970 May;45(2):450–455. doi: 10.1083/jcb.45.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODIN J. Electron microscopy of the kidney. Am J Med. 1958 May;24(5):661–675. doi: 10.1016/0002-9343(58)90373-5. [DOI] [PubMed] [Google Scholar]
- Skoza L., Mohos S. C. Nephritogenic antigenicity of chromatographic fractions of trypsin and pronase glomerular digests. Proc Soc Exp Biol Med. 1969 Sep;131(4):1189–1193. doi: 10.3181/00379727-131-34067. [DOI] [PubMed] [Google Scholar]