Abstract
Mammalian hepatocyte nuclear factor-3 (HNF-3) and the Drosophila homeotic gene fork head proteins are prototypes of an extensive family of cell-specific transcription factors that share homology in the winged helix DNA-binding domain. One of these mammalian family members, HNF-3/fork head homolog-4 (HFH-4), was isolated by PCR amplification of rodent brain cDNA and exhibits abundant expression in the adult bronchiolar epithelium. In this study, we performed in situ hybridization of stage-specific mouse embryos and report on a novel expression pattern of the HFH-4 gene in both the presumptive and differentiated choroid plexus epithelium, which is responsible for the synthesis and secretion of cerebrospinal fluid (CSF) proteins. We also showed that HFH-4 is a potent transcriptional activator in cotransfection assays and defined several protein sequences important for HFH-4 transcriptional activity. We used in vitro DNA-binding site selection with recombinant HFH-4 protein and determined that the HFH-4 protein recognizes the DNA consensus sequences HWDTGTTTGTTTA or KTTTGTTGTTKTW (where H is not G, W is A or T, D is not C, and K is G or T). We used this HFH-4 consensus to identify potential HFH-4 target genes in the choroid plexus epithelium and demonstrated that these promoter sequences bind to recombinant HFH-4 protein in electrophoretic mobility shift assays. Recombinant HFH-4 formed specific protein–DNA complexes with the promoter regions of the human prothrombin, beta amyloid precursor protein, α1-antichymotrypsin, cystic fibrosis transmembrane conductance regulator and rodent α2-macroglobulin, growth hormone receptors, and insulin-like growth factor II genes. Furthermore, we identified putative HFH-4 target genes in the bronchiolar epithelium including the clara cell secretory protein gene and the HNF-3α gene, a winged helix family member involved in the transcriptional regulation of genes in the bronchiolar epithelium. In support of these binding studies, cotransfection assays show that HFH-4 potentiates expression of the HNF-3α and clara cell secretory protein promoter regions.
Keywords: winged helix/fork head domain, cell-specific transcription factor, choroid plexus epithelium, bronchiolar epithelium, mouse embryo
Cellular differentiation results in transcriptional induction of distinct sets of tissue-specific genes whose expression is required for organ function. Tissue-restricted gene expression relies upon combinatorial interactions of multiple cis-acting DNA sequences bound by families of cell-specific nuclear factors (1). One of these regulatory families is represented by the hepatocyte nuclear factor-3 (HNF-3α and β) proteins (2), which mediate the transcription of liver (3) and lung (4–7) specific genes. The HNF-3 proteins bind DNA as a monomer via a homologous winged helix DNA-binding domain (8). Mammalian HNF-3 and Drosophila homeotic fork head (fkh) (9) proteins were the first identified members of a large family of transcription factors that shares homology in the winged helix DNA-binding domain and is involved in differentiation of diverse cellular lineages (3).
Accumulating evidence demonstrates that the winged helix transcription factors are involved in cellular differentiation during embryonic development and organogenesis. The HNF-3α and HNF-3β genes are expressed during the primitive streak stage of mouse embryogenesis (10–12). Targeted disruption of the HNF-3β gene causes an embryonic lethal phenotype that exhibits defects in the formation of notochord, neurotube, somites, and gut endoderm (13, 14). Targeted disruption of other winged helix family members, brain factor-1 and brain factor-2, demonstrates an involvement in morphogenesis of the cerebral hemispheres and in branching of the ureter and renal collecting system respectively (15, 16). Disruption of a winged-helix gene (whn) is responsible for the phenotype of the nude mouse mutation (17). Furthermore, aberrant expression of altered winged helix proteins has also been associated with neoplastic transformations (18–20).
In a previous study we isolated a full-length mouse HNF-3/fkh homolog-4 (HFH-4) cDNA, a winged helix family member encoding a 421 amino acid polypeptide containing several putative transcriptional activation motifs (5). In situ hybridization studies of adult rodent lung showed that the winged helix family members HFH-4 and HNF-3α are coexpressed in the proximal bronchiolar epithelium (5). HFH-4 initiates expression in the proximal bronchial epithelium of embryonic lung at 14.5 day post coitum (p.c.) (21). In this study, we report on a novel expression pattern of the HFH-4 gene in the presumptive choroid plexus epithelium of the mouse embryo and show that its expression continues during the differentiation of the choroid plexus epithelium. We determine the HFH-4 DNA-binding consensus sequence and show that HFH-4 is a potent transcriptional activator. We use the HFH-4 DNA-binding consensus sequence to identify putative target genes in the choroid plexus and bronchiolar epithelium.
MATERIALS AND METHODS
In Situ Hybridization of Stage-Specific Embryos and RNase Protection Assays.
In situ hybridization of paraffin-embedded embryos was performed with 33P-labeled antisense RNA probes generated from a XbaI linearized HFH-4 full-length cDNA pGEM1 (XbaI–SalI) template using SP6 RNA polymerase as described (22). Antisense 33P-labeled RNA probes were hybridized to sectioned dewaxed tissue and rinsed at high stringency followed by autoradiography as described (22). A darkfield condenser was used to enhance the visualization of the silver grains due to specific HFH-4 hybridization. RNase protection assays were performed as described (23). The HFH-4 riboprobe was synthesized using T7 RNA polymerase from an HindIII-digested HFH-4 cDNA template (250 bp EcoRI HFH-4 winged helix fragment subcloned in pGEM1).
In Vitro DNA-Binding Site Selection and Electrophoretic Mobility Shift Assays (EMSA).
HFH-4 winged helix DNA-binding domain (amino acids 110–236) was fused to the glutathione S-transferase (GST) protein and affinity purified from bacterial extracts via glutathione chromatography (24). The purified GST-HFH-4 fusion protein was used to isolate high-affinity HFH-4-binding sites from a pool of partially degenerate oligonucleotides containing 14 degenerate positions (1 μg) by six cycles of repetitive protein selection and PCR amplification as described in Overdier et al. (24). The sequence of the degenerate oligonucleotide is as follows: 5′-GTGAAGCTTGCACGT (N)14 CGAGTCTAGACTC (underlined are the HindIII and XbaI sites). Ninety of the selected binding sites were tested for HFH-4 protein complex formation by EMSA using 60 ng of purified GST-HFH-4 protein and 47 of these sites bound HFH-4 protein with high affinity (24). The frequency of occurrence for each nucleotide was determined from comparison of 47 high-affinity HFH-4 sites and used to compile a 13 nucleotide HFH-4-binding consensus sequence (Table 1; the fourteenth nucleotide position was degenerate). We used the HFH-4 DNA-binding consensus sequence to search 17 promoter regions (extracted from GenBank) of genes expressed in the bronchiolar epithelium and 8 of these promoters contained putative HFH-4-binding sites. We also searched 24 promoter regions of genes expressed in the choroid plexus epithelium and 8 of these promoters contained putative HFH-4-binding sites. By contrast, only 2 potential HFH-4 targets out of 21 genes were identified in intestinal epithelium (a cell type that does not express HFH-4). Double-stranded oligonucleotides were made to the potential HFH-4-binding sites in these promoter regions (see Table 3 for the promoter sequences) and to previously described winged helix DNA-binding sites (24). These binding sites were used for EMSA with 60 ng of affinity-purified GST-HFH-4 fusion protein as described (24).
Table 1.
HFH-4 consensus DNA-binding sequence
| G | 9 | — | 42 | 4 | 89 | — | — | — | 94 | — | — | — | 4 |
| A | 51 | 47 | 19 | 13 | 11 | 2 | — | — | 6 | — | 4 | 4 | 87 |
| T | 21 | 53 | 38 | 68 | — | 98 | 100 | 100 | — | 94 | 96 | 96 | 9 |
| C | 19 | — | — | 15 | — | — | — | — | — | 6 | — | — | — |
| Consensus | A | A | T | T | G | T | T | T | G | T | T | T | A |
| t | T | G | |||||||||||
| c | a | ||||||||||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
The HFH-4 DNA binding-consensus sequence is compiled from 47 high-affinity HFH-4 binding sites isolated by in vitro binding site selection using recombinant HFH-4 protein as described (24). Shown is the percent nucleotide occurrence for each nucleotide position in the HFH-4 binding site. Uppercase letters indicate nucleotides that are highly represented in DNA sites and lowercase letters indicate nucleotides that are represented half as frequently. The HFH-4 DNA binding-core sequence is indicated by the underlined nucleotides.
Table 3.
Putative HFH-4 target genes
| Gene | GenBank accession no. | Promoter position | Promoter sequence | HFH-4 binding* |
|---|---|---|---|---|
| Choroid plexus epithelium | ||||
| Human prothrombin | LEFTM17262 | −2013/−2025 | TTTTGTTTGTTTt | +++ |
| Human βAPP | LEFTM24546 | −568/−580 | TTTTGTTTGTTTt | +++ |
| Rat α2-macroglobulin | LEFTM23567 | −2016/−2004 | ATTTGTTTGTTTg | ++ |
| Human α1AC | LEFTX94997 | −1227/−1215 | gTTTGTTTGTTTt | +++ |
| Mouse IGF-II | LEFTM36329 | −701/−713 | CAATGTTTGTTct | + |
| Rat growth horm. Rec.† | LEFTS76627 | −136/−148 | TTTTGTTGTTgTt | +++ |
| Bronchiolar epithelium | ||||
| Human CFTR†‡ | LEFTM58478 | −1739/−1751 | ATcTGTTGTTTgG | + |
| Rat HNF-3α | LEFTU86584 | −465/−477 | CTTTGTTTacaaA | ++ |
| Rat CCSP | LEFTX51318 | −1728/−1716 | TTTTGTTTGTTTg | ++ |
| Mouse CCSP | LEFTL24372 | −2072/−2060 | TTTTGTTTGTTTg | ++ |
| Rat HNF-3β | LEFTU50407 | −97/−85 | CccTGTTTGTTTT | — |
| HFH-4 consensus: | HWDTGTTTGTTTA | |||
βAPP, β amyloid precursor protein; α1AC, antichymotrypsin; IGF-II, insulin-like growth factor-II; horm. rec., hormone receptor; CFTR, cystic fibrosis transmembrane conductance regulator; and CCSP, clara cell secretory protein genes.
HFH-4 protein binding affinity is as follows: +++, high; ++, moderate; +, weak; −, none.
Homology with the HFH-2 no. 7 sequence (Table 2), which binds HFH-4 protein with high affinity.
CFTR is expressed in bronchiolar and choroid plexus epithelium. Nucleotides which deviate from the HFH-4 consensus or HFH-2 no. 7 sequence are indicated in lowercase letters.
Construction of HFH-4 cDNA Deletions, Cotransfection Assays, and Analysis of Protein Production via Western Blot Analysis.
To determine the HFH-4 transcriptional activity, cotransfection assays were performed in human hepatoma HepG2 cells, a cell line that does not express endogenous HFH-4 protein. This cotransfection assay consisted of a reporter plasmid containing four copies of the HFH-4 no. 3 binding site (Table 2) upstream of a TATA box-driven luciferase gene (pGL2-basic vector, Promega), an expression vector that uses the cytomegalovirus (CMV) promoter to express the HFH-4 cDNA sequences and a CMV promoter-driven β-galactosidase control plasmid to normalize for differences in transfection efficiency (22). The full-length HFH-4 cDNA was cloned as an XbaI–SalI fragment (5′-3′) in the CMV expression vector (23, 25). HFH-4 cDNA deletions were created by a PCR-mediated strategy using Vent DNA polymerase (New England Biolabs) as described (23).
Table 2.
HFH-4 binding activity
| Binding site | Sequence | HFH-4 Binding* |
|---|---|---|
| 1. HFH-8 | ATGTGTTTGTTTA | +++ |
| 2. HNF-3 no. 4 | CAATGTTTGTTTA | +++ |
| 3. HFH-2 no. 7 | gTTTGTTGTTTTA | +++ |
| 4. HFH-2 no. 12 | TATTGTTaTTTTA | + |
| 5. HFH-4 no. 3 | gTTTGTTTacaat | ++ |
| 6. HNF-3 no. 4m1a | CAATaTTTGTTTA | − |
| 7. HNF-3 no. 4m2a | CAATaTTTaTTTA | − |
| 8. Ceruloplasmin | CAGaGTTTGTgTg | − |
| 9. TTF-1 | TcGcGTTTGTTTt | − |
| HFH-4 Consensus: | HWDTGTTTGTTTA |
Isolation of HFH-2 and HNF-3 DNA binding sequences was previously (24). Nucleotides which deviate from the HFH-4 consensus or HFH-2 no. 7 sequence are indicated in lowercase letters. Nucleotide abbreviations are as follows: H is not G, W is A or T and D is not C. Ceruloplasmin, ceruloplasin −245/−233; TTF-1, thyroid transcription factor-1 −196/−208.
HFH-4 protein binding affinity is as follows: +++, high; ++, moderate; +, weak; −, none.
Human hepatoma HepG2 cells were maintained in monolayer cultures and transfected (23, 25) using Lipofectin reagent (GIBCO/BRL) according to manufacturer’s protocol (22-mm plates/200 ng CMV-HFH-4 expression vector/800 ng of 4× HFH-4 no. 3 luciferase reporter/40 ng CMV-β-galactosidase construct/4 μl lipofectin). Cellular protein extracts were prepared from transfected cells 48 hr after transfection and analyzed for luciferase enzyme activity using a commercially available luciferase assay system (Promega). β-galactosidase enzyme activity was determined as described (23, 25). For the HFH-4 cotransfection assay with target promoters, we used the dual luciferase reporter assay system (Promega), which employs the CMV-renilla luciferase plasmid as a positive control and firefly luciferase as the reporter. HepG2 cells were plated on 22-mm well plates and transfected with Lipofectin reagent using 700 ng of target promoter firefly luciferase construct, 100 ng of CMV-HFH-4 expression vector, and 25 ng of the CMV-renilla luciferase control plasmid. Transfected cellular extracts were assayed for firefly and renilla luciferase enzyme activity as described by the manufacturer (Promega).
For the generation of an HFH-4 specific antibody, HFH-4 N-terminal sequences (amino acids 1–101) were fused to the GST: recombinant HFH-4 protein was isolated from bacterial cultures, purified to homogeneity via glutathione-affinity chromatography (24), and used to immunize rabbits (antisera was generated by the Animal Facility of the University of Illinois at Chicago). Affinity purification of HFH-4 antisera was performed as described (26). To determine the expression of HFH-4 deletion mutants during cotransfection experiments, nuclear extracts were prepared from HepG2 cells transfected with the HFH-4 cDNA constructs and analyzed by Western blotting with HFH-4 antisera as described (23).
RESULTS
The HFH-4 Gene Is Expressed in the Presumptive Choroid Plexus Epithelium.
To determine embryonic expression patterns of the HFH-4 gene, we performed in situ hybridization of mouse embryo paraffin sections with a 33P-labeled antisense HFH-4 RNA probe (Fig. 1). After hybridization, stringent washes, and autoradiography, darkfield microscopy was used to visualize HFH-4 expressing cells in the tissues. In 11 day p.c. mouse embryos, HFH-4 displays expression in ependymal (epithelial) cells lining the ventricles, which constitute the presumptive choroid plexus epithelium (Fig. 1A). The choroid plexus is a vascular structure that lines the ventricles of the brain and is responsible for the synthesis and secretion of the CSF proteins. No in situ hybridization signals were observed in the embryos when the HFH-4 sense riboprobe was used (data not shown). Abundant HFH-4 expression continues in the choroid plexus of the fourth and lateral ventricle and in the ependymal cells lining the mesencephalic vesicle of 13.5 day p.c.mouse embryos (Fig. 1 B and C). Higher magnification of the choroid plexus in the fourth ventricle demonstrates that HFH-4 expression is restricted to the epithelium of the choroid plexus (Fig. 1D). Consistent with previous RNase protection studies, the HFH-4 gene is not expressed in the proximal bronchial epithelium of 13.5 day p.c. mouse embryo lungs (Fig. 1B), but its expression initiates in these cells 1 day later (21). In situ hybridization studies of transverse sections of embryonic brain reveal extensive HFH-4 expression in the choroid plexus of the lateral, third and fourth ventricles of 12.5- to 14.5 day p.c. mouse embryos (Fig. 2 and data not shown). RNase protection assays with adult rat choroid plexus RNA demonstrate that expression of the HFH-4 gene continues in the adult choroid plexus (data not shown).
Figure 1.
In situ hybridization of mouse embryos shows HFH-4 expression in the choroid plexus epithelium. In situ hybridization of sagittal sections of paraffin-embedded mouse embryo with 33P-labeled antisense HFH-4 RNA probe. (A) HFH-4 expression in 11 day p.c. mouse embryo. The darkfield panel demonstrates that HFH-4 expression (white) is restricted to the neuroepithelium lining the roof of the hindbrain (RHB) in the fourth ventricle, in regions separating the mesencephalic vesicle (MV), and telencephalic vesicle (TV) and in the floorplate (Fp) of the neural lumen (NL). Indicated are other structures that do not express HFH-4 including nasal cavity (NC), oropharynx (Op), heart (He), liver (Li), and hindlimb (HL). (B) HFH-4 expression in 13.5 day p.c. mouse embryo. HFH-4 is expressed in the choroid plexus (CP) epithelium of the fourth ventricle (FV) and lateral ventricle (LV) and in the epithelium lining part of the mesencephalic vesicle (MV). No HFH-4 expression is observed in lung (Lu), upper lip (UL), lower lip (LL), vertebrae (Ve), and pericardial cavity (PC). (C) Enlargement of 13.5 day p.c. embryonic brain shows that HFH-4 expression is restricted to the choroid plexus epithelium and ependymal (epithelial) cells lining the lateral, mesencephalic, and fourth ventricles. (D) Enlargement of the fourth ventricle of 13.5 day p.c. embryonic brain shows HFH-4 expression in the choroid plexus epithelium.
Figure 2.
In situ hybridization of mouse embryonic brain demonstrates HFH-4 expression in the choroid plexus epithelium. In situ hybridization of transverse sections of embryonic head region with 33P-labeled antisense HFH-4 RNA probe. (A) HFH-4 expression in 14.5 day p.c. mouse embryonic brain. HFH-4 expression is found in the choroid plexus (CP) epithelium and ependymal cells of the fourth ventricle (FV) and lateral ventricle (LV). (B) HFH-4 expression in 13.5 day p.c. mouse embryonic brain. HFH-4 expression is found in the choroid plexus epithelium and ependymal cells of the fourth ventricle but not in the nasal cavity (NC) epithelium or the lens (L) and epithelium of the eye. (C) HFH-4 expression in 12.5 day p.c. mouse embryonic brain. HFH-4 expression is found in the choroid plexus epithelium of the fourth, lateral, and third ventricle (ThV or mesencephalic vesicle) of the brain.
Determination of the HFH-4 DNA-Binding Consensus Sequence.
To identify putative target genes expressed in bronchiolar and choroid plexus epithelium, we determined the HFH-4 DNA-binding consensus sequence using a process of repetitive protein selection and PCR amplification from a population of degenerate double-stranded oligonucleotides as described (24). Forty-seven high-affinity HFH-4-binding sequences, as evidenced by protein–DNA complex formation in EMSA, were compared to determine the percent occurrence for each nucleotide position (Table 1). This comparison allowed us to compile the HFH-4-binding consensus sequence (HWDTGTTTGTTTA; where H is not G, W is A or T, and D is not C). DNA sites containing either the TGTTTGT or TGTTGTT core sequence, which did not deviate significantly from the HFH-4 consensus sequence, formed abundant HFH-4 protein–DNA complexes in EMSA (Table 2, sequences 1–3; data not shown). Reduced or no HFH-4-binding activity was exhibited by sequences that substituted the G residues in the core sequence with A residues or possessed nucleotide changes from the consensus outside the core sequence (Table 2, sequences 4–9). Thus, the DNA-binding specificity of the HFH-4 protein is more restricted than that of other winged helix family members (24, 27).
The HFH-4 Protein Is a Potent Transcriptional Activator.
The HFH-4 protein contains regions homologous to other transcriptional activation motifs (5, 21). These include four regions that are rich in acidic amino acid residues (A1–A4), a proline-rich region (28), and the winged helix transcriptional activation region II motif (25). To determine whether these HFH-4 protein sequences contribute to transcriptional activation, cotransfection assays (23) were employed to compare wild-type HFH-4 protein with truncated HFH-4 proteins deleted in these regions (see Materials and Methods). To avoid complications with endogenous HFH-4 protein, we chose the human hepatoma HepG2 cell line that does not express HFH-4 to perform the cotransfection assays. Cotransfection assays with the full-length HFH-4 cDNA provided a robust 40-fold increase in reporter gene transcription compared with the CMV construct without any insert. This activation was dependent on retention of the HFH-4 recognition sequence in the reporter construct (data not shown). Carboxyl-terminal deletions, which removed the fourth acidic domain (A4), caused an approximately 25% decrease in HFH-4 transcriptional activation (Fig. 3), but no further reduction in activity was observed with C-terminal deletions, which retained the A3 acidic amino acid sequences (data not shown). Carboxyl-terminal deletions that extended through the A3 acidic domain (amino acids 297–331) significantly reduced HFH-4 transcriptional activation and removal of A2 acidic sequences caused no further decreases in activity (Fig. 3). Previous studies demonstrated that mutations of the region II sequences in the HNF-3β protein resulted in significant decreases in transcriptional activation (25). Amino-terminal deletions that removed both region II sequences and the first acidic amino acid region (A1) resulted in a 25% decrease in transcriptional activity (Fig. 3). Removal of amino-terminal sequences extending through the proline-rich amino acid region (amino acids 1–110) eliminated detectable HFH-4 transcriptional activity. We anticipated that the HFH-4 deleted proteins were targeted to the nucleus because they all retained the winged helix domain, which contains the signals necessary for nuclear localization (23). Western blot analysis confirmed that the HFH-4 protein deletions were found in the nuclear extracts prepared from CMV-HFH-4 transfected HepG2 cells (data not shown). These deletions indicated that retention of acidic amino acid regions A1–A3, region II sequences, and the proline-rich sequences are required for minimal transcriptional activation. However, our analysis does not rule out the possibility that HFH-4 deletions influence protein folding.
Figure 3.
Identification of HFH-4 transcriptional activation protein motifs. Schematically shown is the position of potential HFH-4 transcriptional activation motifs of the wild-type HFH-4 protein (5) and truncated HFH-4 proteins synthesized by deleted HFH-4 cDNA constructs. Numbers represent the amino acid positions of the potential transcriptional activation motifs and the winged helix DNA-binding domain. Summarized in the bar graph is the normalized transcriptional activity of the HFH-4 protein deletions relative to the wild-type activity and the error bars represent the SD from three separate experiments. Western blots using an HFH-4 antibody specific to the N terminus of the protein (amino acids 1–100) showed that HFH-4 protein was expressed in nuclear extracts (data not shown).
Identification of Putative Target Genes in the Choroid Plexus and Bronchiolar Epithelium.
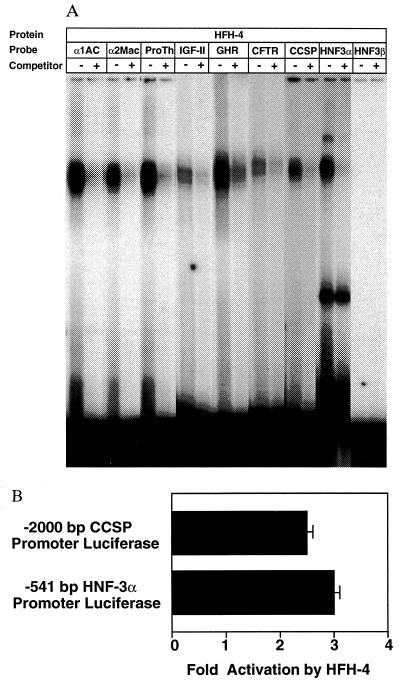
The choroid plexus epithelium is responsible for expression and secretion of the CSF proteins (29). To identify potential HFH-4 target genes in the choroid plexus epithelium, we used the HFH-4 DNA-binding consensus sequence to search promoter regions of 24 genes expressed there. Putative HFH-4-binding sites (Table 3) were found in the promoter regions of the human prothrombin, β-amyloid precursor protein (βAPP), α1AC, cystic fibrosis transmembrane conductance regulator (CFTR), and rat α2-macroglobulin (29, 30), the rodent growth hormone receptor, and IGF-II genes (31–33). To determine whether HFH-4 recognized these regulatory sequences, we synthesized oligonucleotides corresponding to these promoter sequences and used them in EMSA with recombinant HFH-4 protein. Abundant HFH-4 protein–DNA complex formation was observed with HFH-4-binding sites derived from promoter regions of the prothrombin, α2-macroglobulin, α1AC, and growth hormone receptor genes (Fig. 4). The HFH-4 protein-binding sequence in the βAPP promoter region is identical to the prothrombin promoter sequence that binds HFH-4 protein in EMSA. Furthermore, the HFH-4 protein formed specific protein–DNA complexes with the CFTR and IGF-II promoter sites, but exhibited lower affinity than these other promoter sites (Fig. 4A).
Figure 4.
Identification of potential HFH-4 target genes. (A) DNA-binding assays with recombinant HFH-4 protein and potential HFH-4-binding sites in target genes of the choroid plexus and bronchiolar epithelium. EMSA with labeled HFH-4-binding site oligonucleotides (1 ng) and recombinant GST-HFH-4 winged helix protein (60 ng; see Table 3 for sequences). Competitions included a 200-fold molar excess of unlabeled oligonucleotides in the binding reaction. α2MAC, α2-macroglobulin; ProTh, prothrombin; and GHR, growth hormone receptor. Note that the HFH-4-binding sequence in the promoter of the prothrombin gene is identical to that of the βAPP gene (Table 3). (B) Cotransfection assays demonstrate HFH-4 transcriptional activation of putative target promoters. The CMV-HFH-4 expression vector was cotransfected with the −541 rat HNF-3α and the −2000 rat CCSP promoter luciferase plasmids in HepG2 cells and analyzed for luciferase enzyme activity. Shown is the normalized fold promoter activation by the CMV-HFH-4 expression vector compared with the CMV plasmid and the error bars represent the SD from three separate experiments. The −431 rat HNF-3α promoter construct, which lacks the HFH-4-binding site, was not activated by HFH-4.
Similar searches were also performed for HFH-4 target genes in the bronchiolar epithelium. An HFH-4-binding site was found in the CCSP gene and in the functional promoters of the HNF-3α and HNF-3β genes (34, 35), winged helix family members, which are important for regulating pulmonary epithelial-specific gene expression (4–7). DNA-binding assays show that the HFH-4 protein forms abundant complexes with the CCSP and HNF-3α promoter sequences but not with the HNF-3β promoter site, which possessed nucleotide substitutions that are not tolerated for HFH-4 binding (Fig. 4A). Cotransfection studies in HepG2 cells demonstrated that HFH-4 protein potentiated expression of the HNF-3α and CCSP promoter luciferase constructs (Fig. 4B), suggesting that HFH-4 plays an important role in differentiation of bronchiolar epithelium.
DISCUSSION
The winged helix proteins are a large family of transcription factors that share homology in the winged helix DNA-binding domain and are involved in the differentiation of diverse cellular lineages (for review see ref. 3). One of these family members, HFH-4, is expressed in the proximal bronchiolar epithelium beginning at 14.5 day p.c. in mouse embryos, and continuing throughout adulthood in the rodent (5, 21). In this study, we report on a novel expression pattern for the HFH-4 gene in choroid plexus primordia lining the ventricles of the embryonic mouse brain, which continues through the formation and differentiation of the choroid plexus epithelium in embryonic and adult brain. We showed that the HFH-4 protein is a potent transcriptional activator and used the HFH-4 consensus-binding sequence to identify potential HFH-4 target genes in the choroid plexus and bronchiolar epithelium. Deletion analysis determined that HFH-4 transcriptional activity requires N-terminal regions rich in acidic and proline amino acids (28), a region II transcriptional activation motif shared among winged helix transcription factors (25), and two C-terminal acidic amino acid regions lying adjacent to the winged helix DNA-binding domain.
The choroid plexus is a highly vascularized structure lining the ventricles of the brain. Its epithelium forms tight cell junctions, creating part of the blood-brain barrier, and it synthesizes and secretes most of the CSF proteins (29). The presence of HFH-4-binding sites in the promoter regions of the prothrombin, α2-macroglobulin, and α1AC genes suggests that HFH-4 may be involved in regulating their expression and secretion from choroid plexus epithelium into the CSF. The growth hormone receptor gene is also a potential HFH-4 target which functions in transporting growth hormone across the epithelial cell barrier of the choroid plexus epithelium (32, 33). Furthermore, the βAPP gene is an important potential HFH-4 target gene because an increase in its expression is found in the choroid plexus of patients suffering from Alzheimer disease (36). HFH-4 may also regulate CFTR expression, which functions to regulate electrolyte homeostasis and thus CSF secretion from the choroid plexus epithelium (30). Mutations in the CFTR gene result in extensive cystic fibrosis associated pathologies because of impaired regulation of electrolyte and fluid homeostasis in the lung (37). Moreover, HFH-4 expression in the choroid plexus epithelium precedes that of its potential target gene, IGF-II, which is a CSF growth factor that influences the proliferation of neuroepithelium during embryonic development (38). Both the expression of HFH-4 in the presumptive choroid plexus epithelium and identification of target genes in this cell type leads us to the hypothesis that the HFH-4 protein is important for the differentiation of the choroid plexus epithelium.
Analysis of promoters regulating liver-enriched transcription factors suggests that expression in hepatocytes is maintained through cross-regulation of the promoter by one or more unrelated liver-enriched transcription factors. A cross-talk regulatory loop was discovered during the analysis of the HNF-1 and HNF-4 promoters in which each of these factors regulated the transcription of the other promoter (39, 40). HNF-3β expression is also regulated by basic region leucine zipper and proline and acidic amino acid-rich basic region leucine zipper family members and by HNF-6 (34, 41, 42). Cross-regulatory mechanisms may also play a role in maintaining cell-specific transcription factor expression in the respiratory epithelium. Recent studies have implicated the HNF-3 proteins in regulating expression of thyroid transcription factor-1 in the respiratory epithelium (6). Our current studies extend these results by demonstrating that HFH-4 protein binds to and activates the expression of the HNF-3α promoter region and may therefore mediate cross-regulation of HNF-3α gene expression in respiratory epithelium. However, HFH-4 exhibits selective protein recognition since it does not bind to the autoregulatory-binding site in the HNF-3β promoter region (34), which is also expressed in bronchiolar epithelium (43). Thus, HNF-3α promoter regulation by HNF-3β and HFH-4 may provide a mechanism to drive abundant HNF-3α transcription in bronchiolar epithelium.
We also have identified an HFH-4-binding site within the regulatory region required for appropriate CCSP expression in proximal bronchiolar epithelium of transgenic mice, which displays sequence conservation between rat and mouse promoters (44). Expression of HFH-4 precedes that of the CCSP gene (43), which is transcribed during terminal differentiation of proximal respiratory epithelium (5, 21). The fact that HFH-4 binds to and activates the CCSP and the HNF-3α promoter regions lends credence to the hypothesis that HFH-4 may participate in terminal differentiation of bronchiolar epithelium during development. Further experiments involving ectopic expression of HFH-4 in transgenic mice and targeted HFH-4 gene disruption in mice using embryonic stem cell technology may provide in vivo models to address whether HFH-4 is a determinant of the differentiation of bronchiolar and choroid plexus epithelium.
Acknowledgments
We thank P. Raychaudhuri, K. Colley, and U. Samadani for critically reading the manuscript and C. Butler and R. Qian for their assistance with the GST-HFH-4 fusion protein. We are grateful to S. Duncan for teaching us the in situ hybridization protocol. We thank A. Porcella for the rat choroid plexus RNA and J. A. Whitsett for the rat −2000 bp CCSP promoter luciferase construct. This work was supported by a research grant from The Council for Tobacco Research–USA (2822AR2). R.H.C. is an Established Investigator of the American Heart Association/Bristol–Myers Squibb.
ABBREVIATIONS
- HNF-3
hepatocyte nuclear factor 3
- HNF-4
HNF-3/fork head homolog-4
- CMV
cytomegalovirus
- GST
glutathione S-transferase
- p.c.
post coitum
- EMSA
electrophoretic mobility shift assay
- CCSP
clara cell secretory protein
- IGF-II
insulin-like growth factor II
- CSF
cerebrospinal fluid
- α1AC
α1-antichymotrypsin
- βAPP
β-amyloid precursor protein
- CFTR
cystic fibrosis transmembrane conductance regulator
References
- 1.Zaret K S. Annu Rev Physiol. 1996;58:231–251. doi: 10.1146/annurev.ph.58.030196.001311. [DOI] [PubMed] [Google Scholar]
- 2.Lai E, Prezioso V R, Tao W F, Chen W S, Darnell J E., Jr Genes Dev. 1991;5:416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- 3.Costa R H. In: Liver Gene Expression. Tronche F, Yaniv M, editors. Austin, TX: R. G. Landes; 1994. pp. 183–206. [Google Scholar]
- 4.Bohinski R J, Di Lauro R, Whitsett J A. Mol Cell Biol. 1994;14:5671–5681. doi: 10.1128/mcb.14.9.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clevidence D E, Overdier D G, Peterson R S, Porcella A, Ye H, Paulson E K, Costa R H. Dev Biol. 1994;166:195–209. doi: 10.1006/dbio.1994.1307. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Shaw-White J R, Wert S E, Whitsett J A. Mol Cell Biol. 1996;16:3626–3636. doi: 10.1128/mcb.16.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawaya P L, Stripp B R, Whitsett J A, Luse D S. Mol Cell Biol. 1993;13:3860–3871. doi: 10.1128/mcb.13.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark K L, Halay E D, Lai E, Burley S K. Nature (London) 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 9.Weigel D, Jackle H. Cell. 1990;63:455–456. doi: 10.1016/0092-8674(90)90439-l. [DOI] [PubMed] [Google Scholar]
- 10.Ang S L, Wierda A, Wong D, Stevens K A, Cascio S, Rossant J, Zaret K S. Development (Cambridge, UK) 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan A P, Kaestner K H, Grau E, Schutz G. Development (Cambridge, UK) 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki H, Hogan B L. Development (Cambridge, UK) 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Ang S L, Rossant J. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein D C, Ruiz i Altaba A, Chen W S, Hoodless P, Prezioso V R, Jessell T M, Darnell J., Jr Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 15.Xuan S, Baptista C A, Balas G, Tao W, Soares V C, Lai E. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 16.Hatini V, Huh S O, Herzlinger D, Soares V C, Lai E. Genes Dev. 1996;10:1476–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 17.Nehls M, Pfelfer D, Schorpp M, Hedrich H, Boehm T. Nature (London) 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Vogt P K. Proc Natl Acad Sci USA. 1993;90:4490–4494. doi: 10.1073/pnas.90.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Chang H W, Lai E, Parker E J, Vogt P K. Cancer Res. 1995;55:5540–5544. [PubMed] [Google Scholar]
- 20.Fredericks W J, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr F G, Rauscher F., Jr Mol Cell Biol. 1995;15:1522–1535. doi: 10.1128/mcb.15.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackett B P, Brody S L, Liang M, Zeitz I D, Bruns L A, Gitlin J D. Proc Natl Acad Sci USA. 1995;92:4249–4253. doi: 10.1073/pnas.92.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan S A, Manova K, Chen W S, Hoodless P, Weinstein D C, Bachvarova R F, Darnell J E., Jr Proc Natl Acad Sci USA. 1994;91:7598–7602. doi: 10.1073/pnas.91.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian X, Costa R H. Nucleic Acids Res. 1995;23:1184–1191. doi: 10.1093/nar/23.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overdier D G, Porcella A, Costa R H. Mol Cell Biol. 1994;14:2755–2766. doi: 10.1128/mcb.14.4.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pani L, Overdier D G, Porcella A, Qian X, Lai E, Costa R H. Mol Cell Biol. 1992;12:3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob A, Budhiraja S, Qian X, Clevidence D, Costa R H, Reichel R R. Nucleic Acids Res. 1994;22:2126–2133. doi: 10.1093/nar/22.11.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierrou S, Hellqvist M, Samuelsson L, Enerbäck S, Carlsson P. EMBO J. 1994;13:5002–5012. doi: 10.1002/j.1460-2075.1994.tb06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell P J, Tjian R. Science. 1989;245:371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- 29.Aldred A R, Brack C M, Schreiber G. Comp Biochem Physiol B Biochem Mol Biol. 1995;111:1–15. doi: 10.1016/0305-0491(94)00229-n. [DOI] [PubMed] [Google Scholar]
- 30.Hincke M T, Nairn A C, Staines W A. J Neurochem. 1995;64:1662–1668. doi: 10.1046/j.1471-4159.1995.64041662.x. [DOI] [PubMed] [Google Scholar]
- 31.Holm N R, Hansen L B, Nilsson C, Gammeltoft S. Brain Res Mol Brain Res. 1994;21:67–74. doi: 10.1016/0169-328x(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 32.Royster M, Driscoll P, Kelly P A, Freemark M. Endocrinology. 1995;136:3892–3900. doi: 10.1210/endo.136.9.7649097. [DOI] [PubMed] [Google Scholar]
- 33.Thornwall M, Chhajlani V, Le Greves P, Nyberg F. Biochem Biophys Res Commun. 1995;217:349–353. doi: 10.1006/bbrc.1995.2783. [DOI] [PubMed] [Google Scholar]
- 34.Pani L, Qian X B, Clevidence D, Costa R H. Mol Cell Biol. 1992;12:552–562. doi: 10.1128/mcb.12.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson R, Clevidence D E, Ye H, Costa R H. Cell Growth Differ. 1997;8:69–82. [PubMed] [Google Scholar]
- 36.Premkumar D R, Kalaria R N. Ann NY Acad Sci. 1996;777:288–292. doi: 10.1111/j.1749-6632.1996.tb34434.x. [DOI] [PubMed] [Google Scholar]
- 37.Frizzell R A. Am J Respir Crit Care Med. 1995;151:S54–S58. doi: 10.1164/ajrccm/151.3_Pt_2.S54. [DOI] [PubMed] [Google Scholar]
- 38.Ayer-le Lievre C, Stahlbom P A, Sara V R. Development (Cambridge, UK) 1991;111:105–115. doi: 10.1242/dev.111.1.105. [DOI] [PubMed] [Google Scholar]
- 39.Kuo C J, Conley P B, Chen L, Sladek F M, Darnell J, Jr, Crabtree G R. Nature (London) 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 40.Zhong W, Mirkovitch J, Darnell J E., Jr Mol Cell Biol. 1994;14:7276–7284. doi: 10.1128/mcb.14.11.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samadani U, Porcella A, Pani L, Johnson P F, Burch J, Pine R, Costa R H. Cell Growth Differ. 1995;6:879–890. [PubMed] [Google Scholar]
- 42.Samadani U, Costa R H. Mol Cell Biol. 1996;16:6273–6284. doi: 10.1128/mcb.16.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Lim L, Costa R, Whitsett J A. J Histochem Cytochem. 1996;44:1183–1193. doi: 10.1177/44.10.8813084. [DOI] [PubMed] [Google Scholar]
- 44.Hackett B P, Gitlin J D. Am J Respir Cell Mol Biol. 1994;11:123–129. doi: 10.1165/ajrcmb.11.2.8049073. [DOI] [PubMed] [Google Scholar]