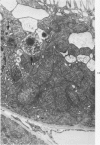
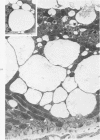
Abstract
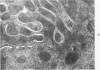
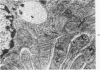
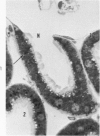
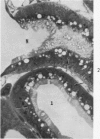
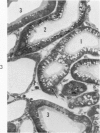
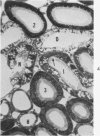
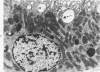
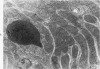
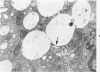
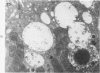
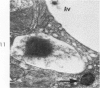
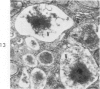
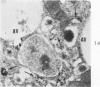
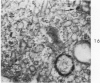
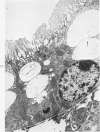
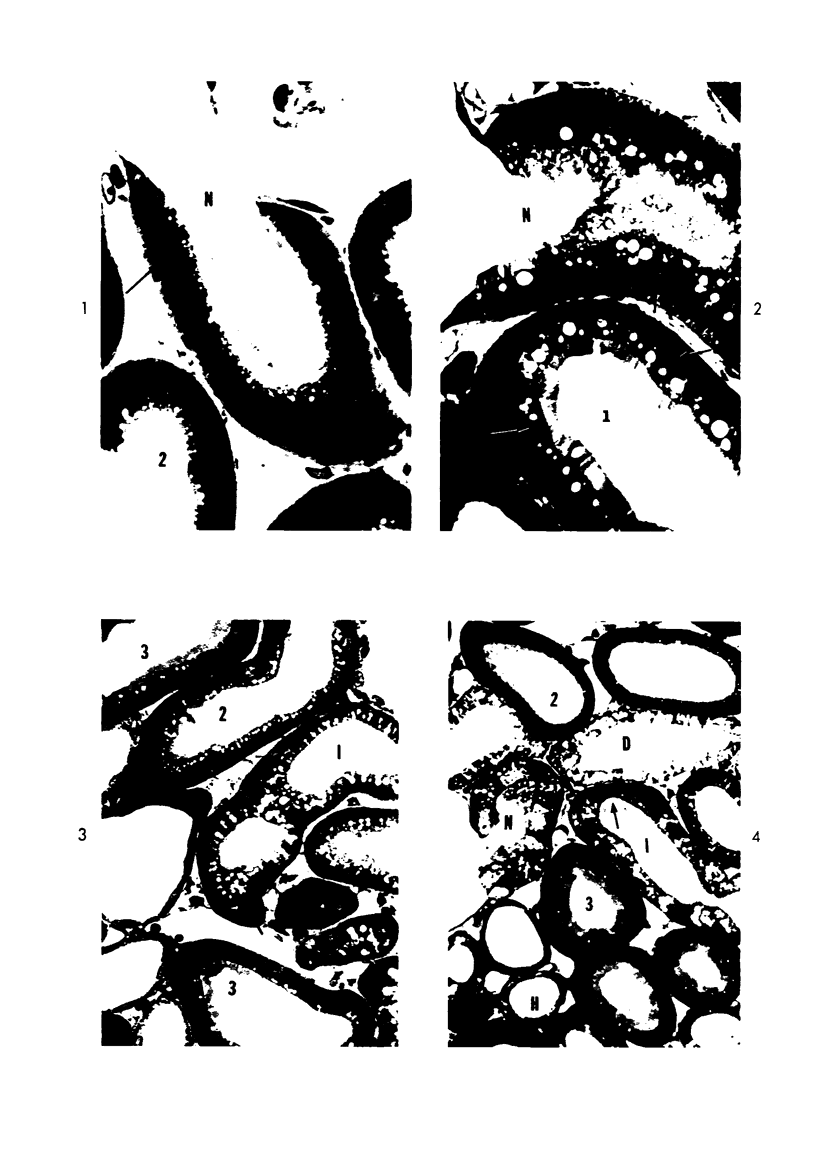
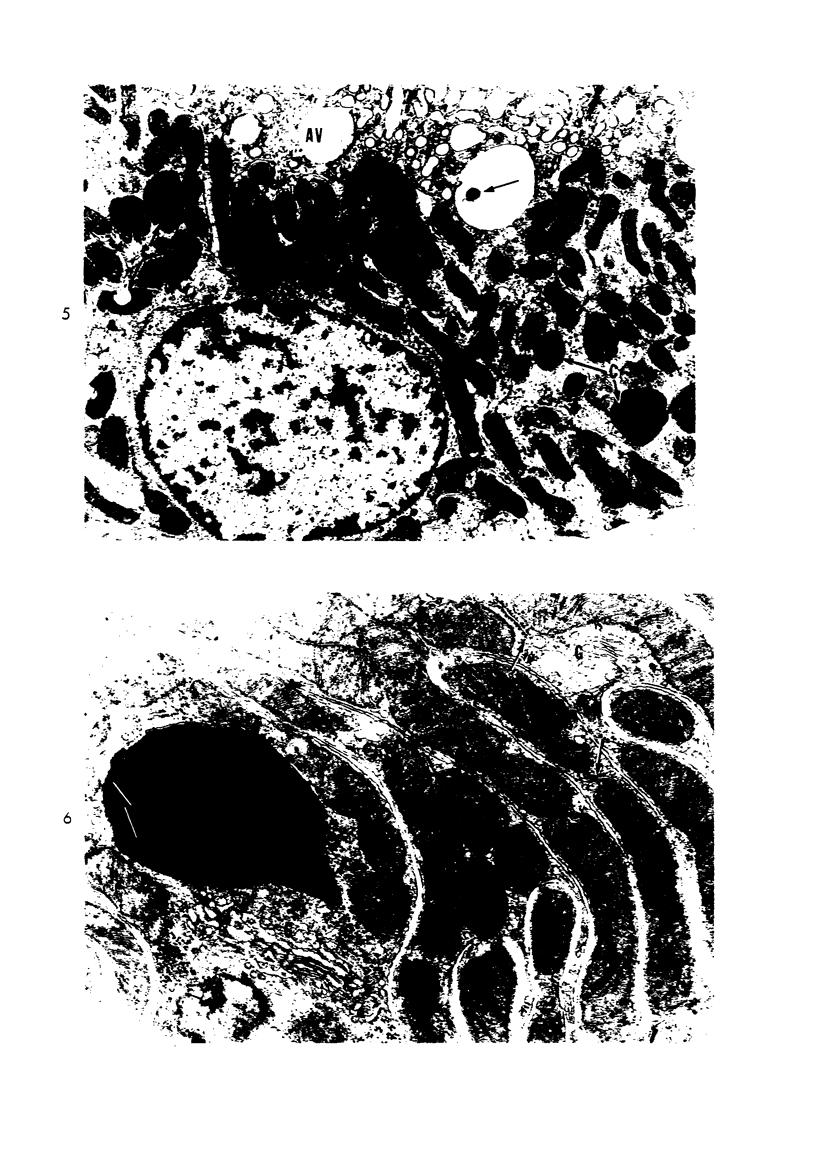
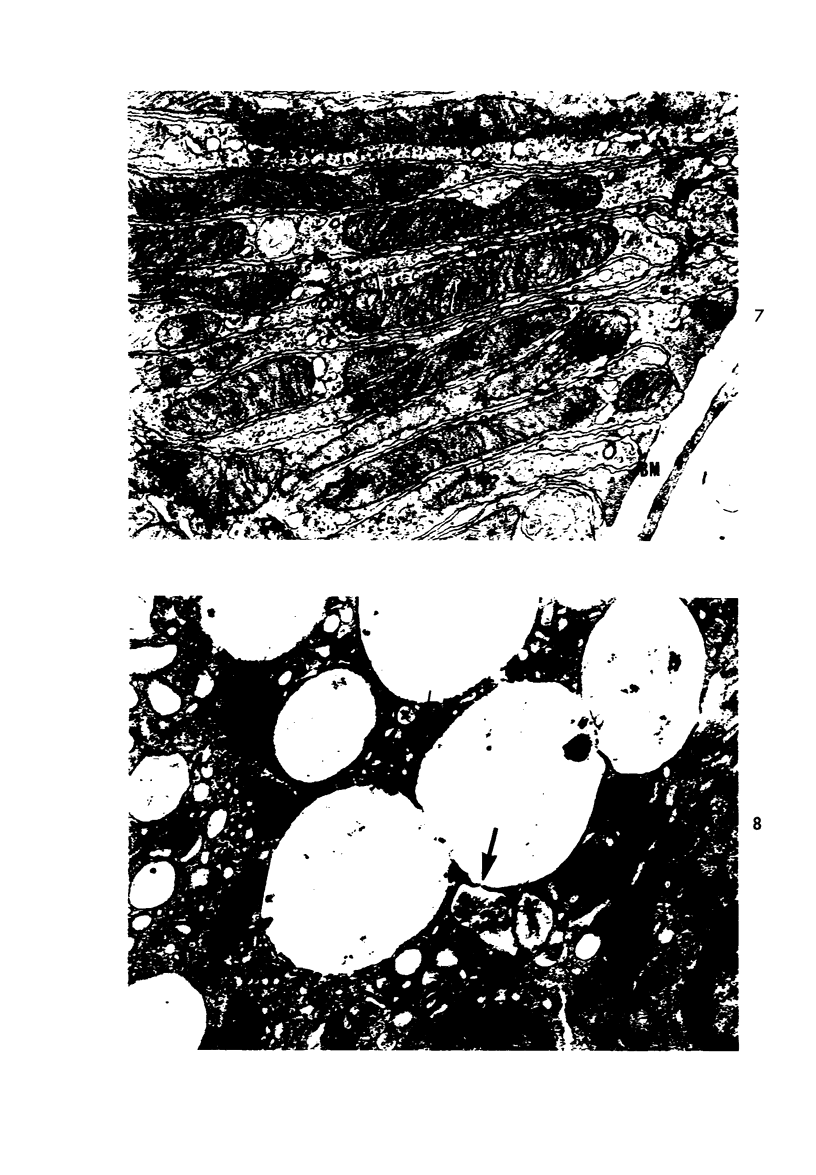
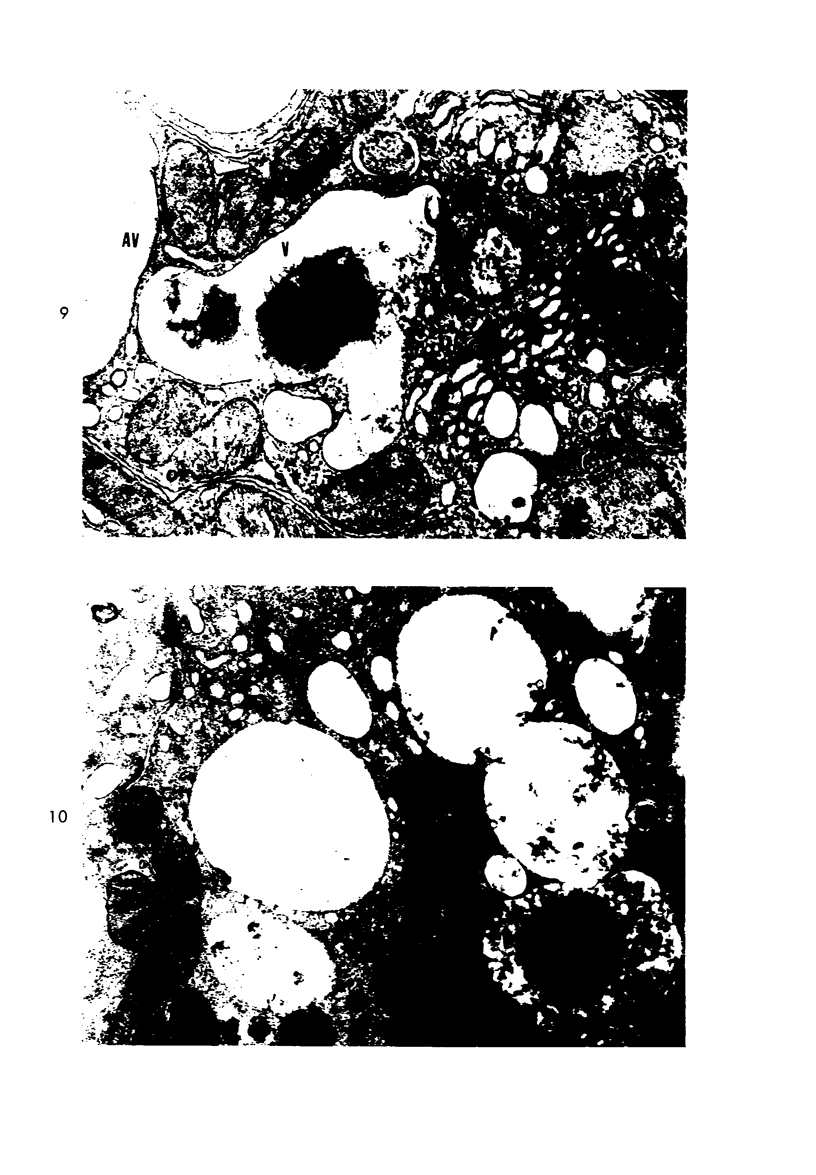
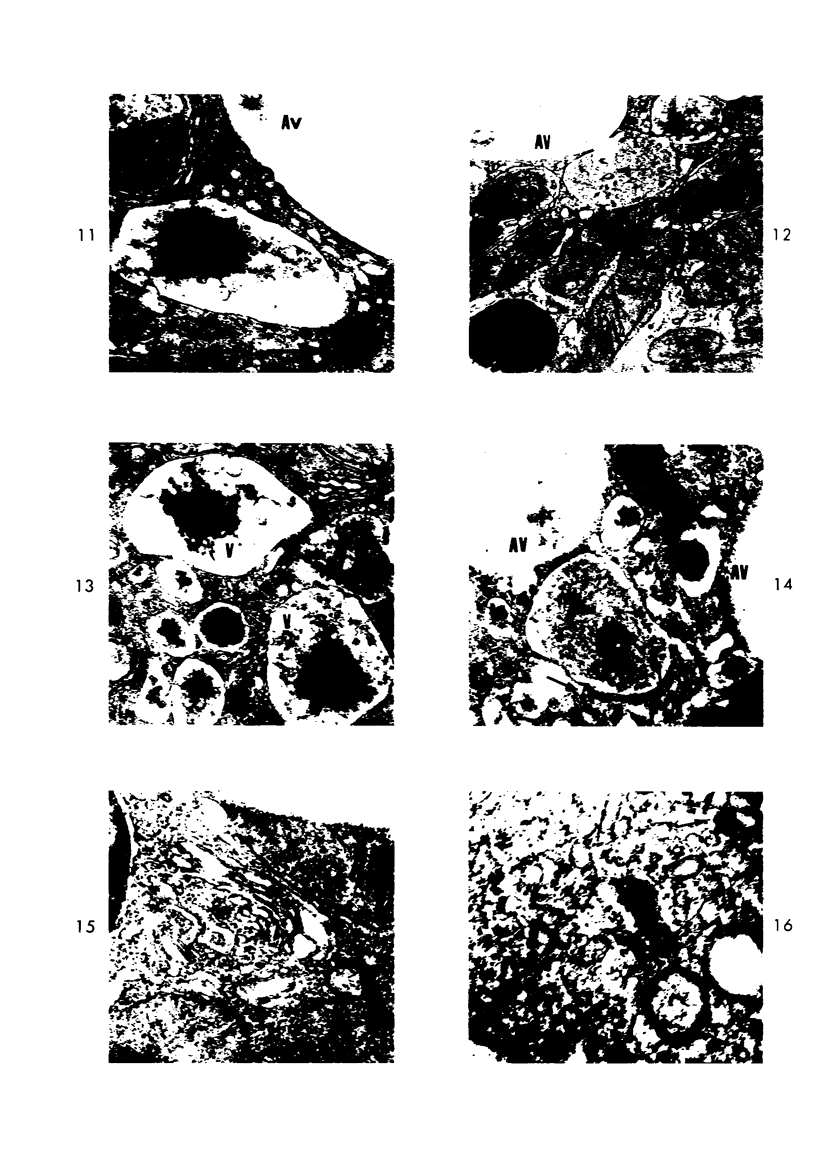
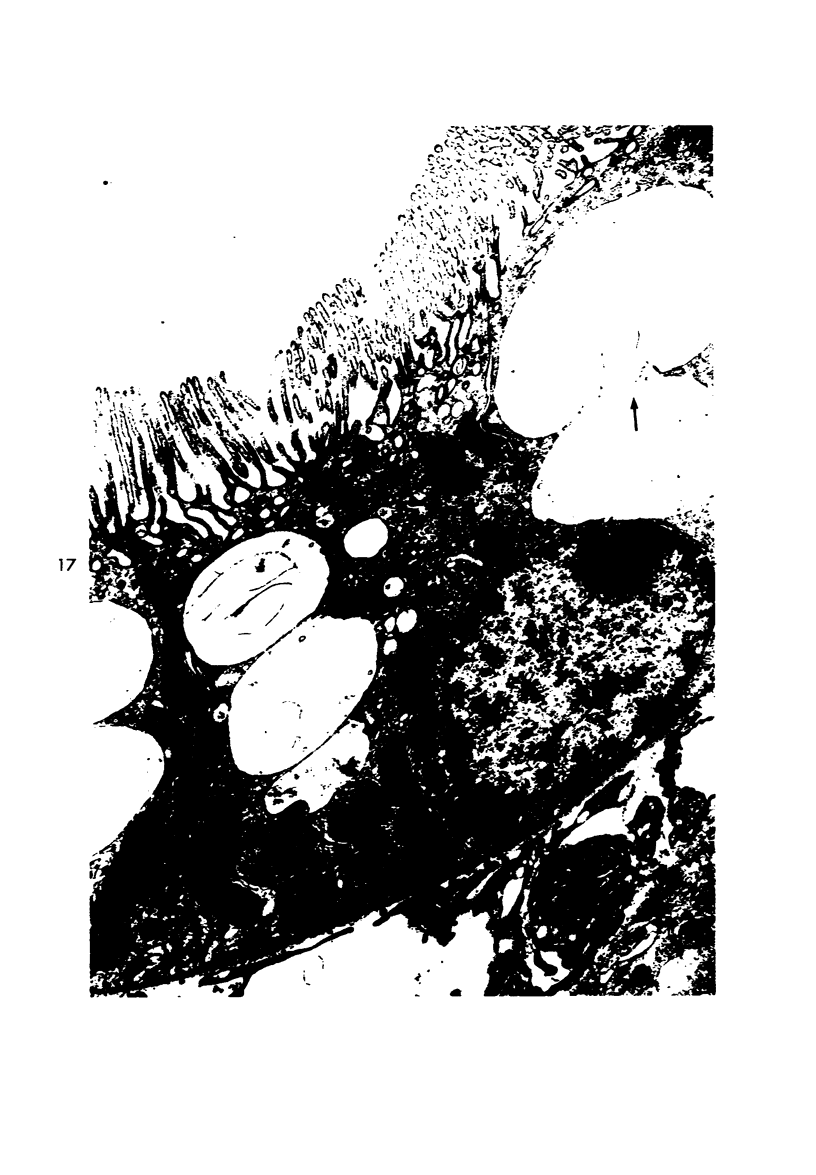
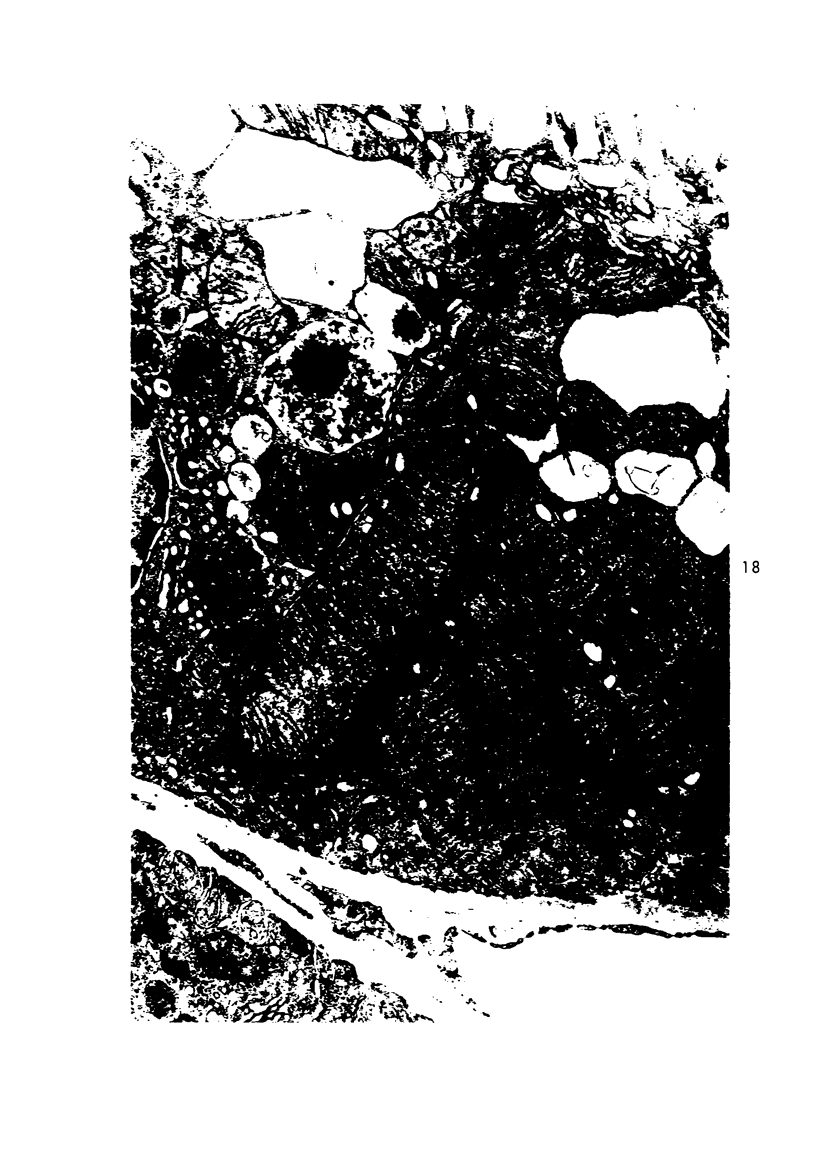
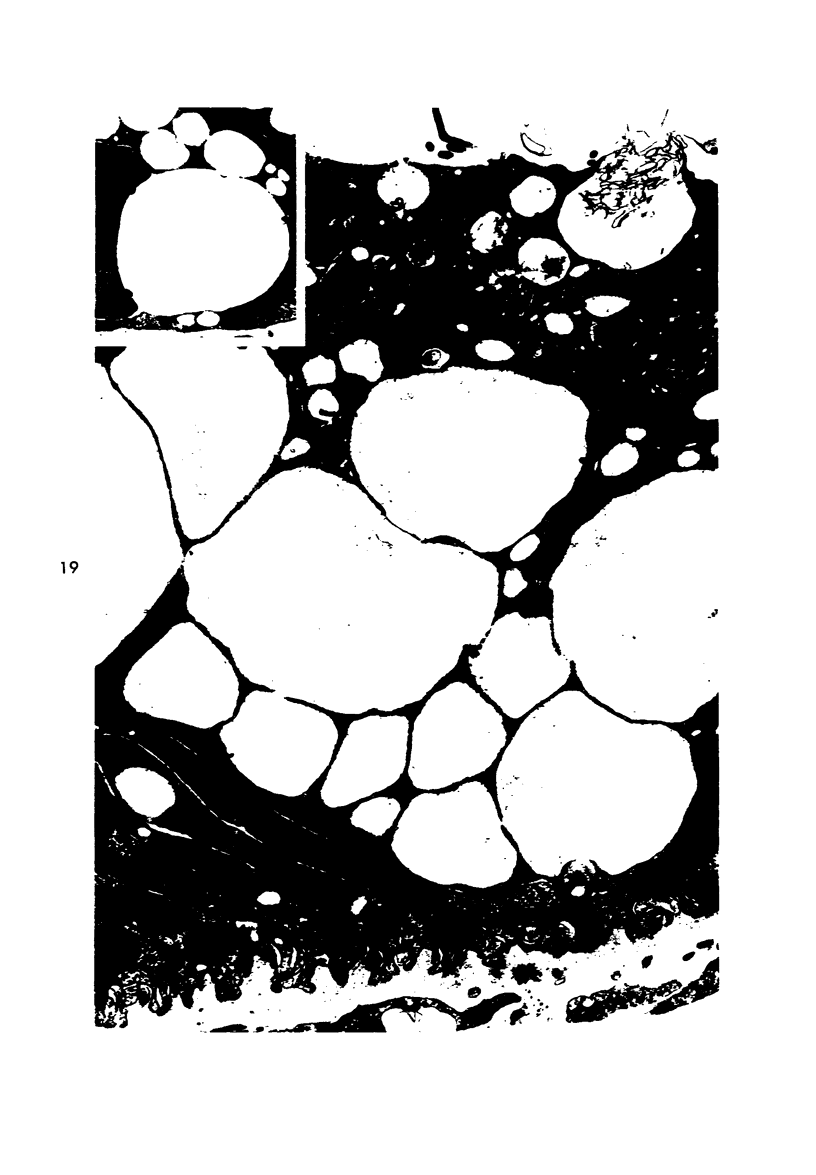
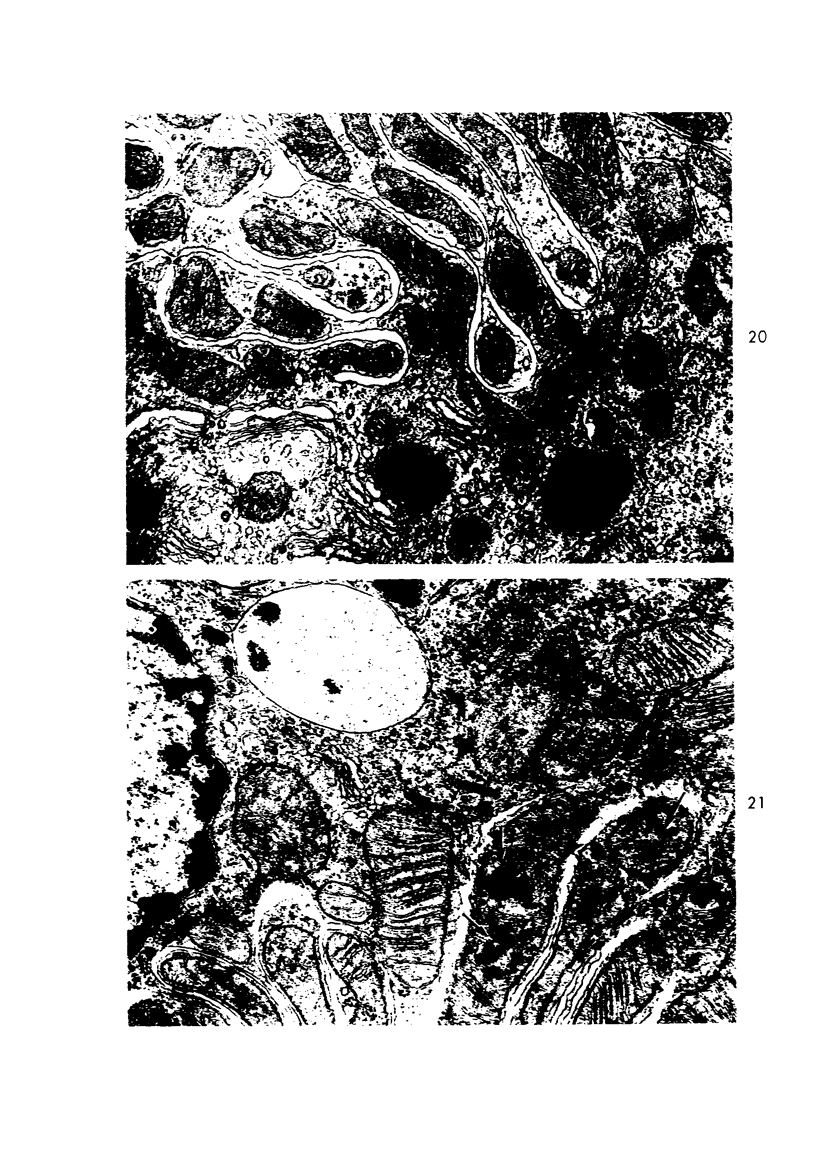
Light and electron microscopic studies of morphologic changes in the rat proximal convoluted tubule after intraperitoneal injection of sodium fluoroacetate (FAc), 60, 20 and 3.5 mg/kg body weight, have been made. Particular attention was directed toward appreciating different changes in the first (S1) and second (S2) segments of the proximal tubule. The earliest change was loss of mitochondrial granules and pallor of the mitochondrial matrix, not necessarily associated with matrix swelling. Matrix swelling was greatest at 3 hours after 3.5 mg/kg and was reversible. However, the mitochondria retained their elongate shape and cristae persisted. At 48 hours, some mitochondria appeared normal; in others, abnormal matrix densities of unknown nature were present. Mitochondrial changes were similar in S1 and S2 at all times. Enlarged apical vacuoles, most pronounced in S1, occurred in all rats after 20 mg/kg. The change was uncommon after 3.5 mg/kg. The hypothesis proposed is that vacuoles arise during an FAc-induced hyperglycemic phase, when pinocytotic activity is maintained but the normal pathway of glucose catabolism is inhibited. Moderate dilatation of the rough-surfaced endoplasmic reticulum occurred during the first 2-hour period in S1 and S2 tubules after high and low doses, but between 6 and 24 hours, dilatation was extensive in S1 tubules after 3.5 mg/kg. This change was reversible. Two types of abnormal vacuolar bodies, large and small, have been described, and were unique to S1 tubules. Acid phosphatase activity was demonstrated in a proportion of the small ones, indiciating that they were a type of lysosome. The larger ones shared features in common with cytosomes of control cells, but acid phosphatase activity was not demonstrated in them and their origins and functions remain obscure. The biochemical lesions induced by fluoroacetate have been discussed and a tentative interpretation of some of the morphologic changes has ben made.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUFFA P., PETERS R. A. The in vivo formation of citrate induced by fluoroacetate and its significance. J Physiol. 1949 Dec;110(3-4):488–500. doi: 10.1113/jphysiol.1949.sp004456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUFFA P., PETERS R. A., WAKELIN R. W. Biochemistry of fluoroacetate poisoning; isolation of an active tricarboxylic acid fraction from poisoned kidney homogenates. Biochem J. 1951 Apr;48(4):467–477. doi: 10.1042/bj0480467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULGER R. E. THE SHAPE OF RAT KIDNEY TUBULAR CELLS. Am J Anat. 1965 Jan;116:237–255. doi: 10.1002/aja.1001160112. [DOI] [PubMed] [Google Scholar]
- Beams H. W., Kessel R. G. The Golgi apparatus: structure and function. Int Rev Cytol. 1968;23:209–276. doi: 10.1016/s0074-7696(08)60273-9. [DOI] [PubMed] [Google Scholar]
- Bovis R., Kasten F. H., Okigaki T. Electron microscopic study of the toxic effect of sodium fluoroacetate on rat myocardial cultures. Exp Cell Res. 1966 Oct;43(3):611–621. doi: 10.1016/0014-4827(66)90032-2. [DOI] [PubMed] [Google Scholar]
- Bowman R. H. Inhibition of citrate metabolism by sodium fluoroacetate in the perfused rat heart and the effect on phosphofructokinase activity and glucose utilization. Biochem J. 1964 Nov;93(2):13C–15C. doi: 10.1042/bj0930013c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B., TRUMP B. F. Correlation of ultrastructure with function in the rat kidney. Am J Pathol. 1962 Feb;40:199–218. [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD M. A. The effects of fluoroacetate, malonate and acid-base balance on the renal disposal of citrate. Biochem J. 1963 Jul;88:115–120. doi: 10.1042/bj0880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A., Granata A. L. Differential toxicity of fluoroacetate to heart, kidney and brain mitochondria of the living rat. Biochem Pharmacol. 1967 Jun;16(6):1083–1089. doi: 10.1016/0006-2952(67)90281-x. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. B., PHILLIPS A. H., Jr Effect of fluoroacetate on glycose metabolism in vivo. Arch Biochem Biophys. 1954 Apr;49(2):389–395. doi: 10.1016/0003-9861(54)90207-6. [DOI] [PubMed] [Google Scholar]
- ENGEL F. L., HEWSON K., COLE B. T. Carbohydrate and ketone body metabolism in the sodium fluoroacetate-poisoned rat; "SFA diabetes". Am J Physiol. 1954 Nov;179(2):325–332. doi: 10.1152/ajplegacy.1954.179.2.325. [DOI] [PubMed] [Google Scholar]
- ERICSSON J. L., TRUMP B. F. ELECTRON MICROSCOPIC STUDIES OF THE EPITHELIUM OF THE PROXIMAL TUBULE OF THE RAT KIDNEY. I. THE INTRACELLULAR LOCALIZATION OF ACID PHOSPHATASE. Lab Invest. 1964 Nov;13:1427–1456. [PubMed] [Google Scholar]
- ERICSSON J. L., TRUMP B. F., WEIBEL J. ELECTRON MICROSCOPIC STUDIES OF THE PROXIMAL TUBULE OF THE RAT KIDNEY. II. CYTOSEGRESOMES AND CYTOSOMES: THEIR RELATIONSHIP TO EACH OTHER AND TO THE LYSOSOME CONCEPT. Lab Invest. 1965 Jul;14:1341–1365. [PubMed] [Google Scholar]
- Ericsson J. L., Trump B. F. Electron microscopic studies of the epithelium of the proximal tubule of the rat kidney. 3. Microbodies, multivesicular bodies, and the golgi apparatus. Lab Invest. 1966 Oct;15(10):1610–1633. [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAL E. M., DREWES P. A., TAYLOR N. F. Metabolism of fluoroacetic acid-2-C-14 in the intact rat. Arch Biochem Biophys. 1961 Apr;93:1–14. doi: 10.1016/0003-9861(61)90308-3. [DOI] [PubMed] [Google Scholar]
- GAL E. M., PETERS R. A., WAKELIN R. W. Some effects of synthetic fluoro compounds on the metabolism of acetate and citrate. Biochem J. 1956 Sep;64(1):161–168. [PMC free article] [PubMed] [Google Scholar]
- GORDON E. E. The metabolism of citrate-C-14 in normal and in fluoroinhibitor-poisoned rats. J Clin Invest. 1961 Sep;40:1719–1726. doi: 10.1172/JCI104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. D., Bulger R. E., Trump B. F. The ultrastructure of the functioning kidney. Lab Invest. 1967 Feb;16(2):220–246. [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C., STRIEBICH M. J. Cytochemical studies. VII. Localization of endogenous citrate in rat liver fractions. J Biol Chem. 1956 Oct;222(2):969–977. [PubMed] [Google Scholar]
- LEHNINGER A. L. Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation. Physiol Rev. 1962 Jul;42:467–517. doi: 10.1152/physrev.1962.42.3.467. [DOI] [PubMed] [Google Scholar]
- LINDENBAUM A., WHITE M. R., SCHUBERT J. Citrate formation in vivo induced by non-lethal doses of fluoroacetate. J Biol Chem. 1951 Jun;190(2):585–593. [PubMed] [Google Scholar]
- LOTSPEICH W. D., PETERS R. A., WILSON T. H. The inhibition of aconitase by 'Inhibitor fractions' isolated from tissues poisoned with fluoroacetate. Biochem J. 1952 Apr;51(1):20–25. doi: 10.1042/bj0510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT R., IKKOS D., PALMIERI G., ERNSTER L., AFZELIUS B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962 Sep;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUNSBACH A. B., MADDEN S. C., LATTA H. Light and electron microscopic changes in proximal tubules of rats after administration of glucose, mannitol, sucrose, or dextran. Lab Invest. 1962 Jun;11:421–432. [PubMed] [Google Scholar]
- MORRISON J. F., PETERS R. A. Biochemistry of fluoroacetate poisoning: the effect of fluorocitrate on purified aconitase. Biochem J. 1954 Nov;58(3):473–479. doi: 10.1042/bj0580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDGE G. H. Electrolyte and water metabolism of rabbit kidney slices; effect of metabolic inhibitors. Am J Physiol. 1951 Oct;167(1):206–223. doi: 10.1152/ajplegacy.1951.167.1.206. [DOI] [PubMed] [Google Scholar]
- Margreth A., Azzone G. F. A study of respiration in fluoroacetate-poisoned muscle preparations. Biochem J. 1964 Jul;92(1):73–82. doi: 10.1042/bj0920073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res. 1966 Oct;16(3):239–258. doi: 10.1016/s0022-5320(66)80060-6. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the ultrastructure and acid phosphatase activity of the cytoplasmic bodies in rat kidney proximal tubule cells. With a comment on their classification. J Ultrastruct Res. 1966 Oct;16(3):197–238. doi: 10.1016/s0022-5320(66)80059-x. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. II. Effects of varying osmolality, ionic strength, buffer system and fixative concentration of glutaraldehyde solutions. J Ultrastruct Res. 1966 Jun;15(3):283–309. doi: 10.1016/s0022-5320(66)80110-7. [DOI] [PubMed] [Google Scholar]
- PETERS R. A. Lethal synthesis. Proc R Soc Lond B Biol Sci. 1952 Feb 28;139(895):143–170. doi: 10.1098/rspb.1952.0001. [DOI] [PubMed] [Google Scholar]
- PETERS R., WAKELIN R. W. Biochemistry of fluoroacetate poisoning; the isolation and some properties of the fluorotricarboxylic acid inhibitor of citrate metabolism. Proc R Soc Lond B Biol Sci. 1953 Jan 15;140(901):497–507. doi: 10.1098/rspb.1953.0004. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., BUSCH H., BOTHWELL J. Method for the study of tissue metabolism in vivo using fluoracetate. Proc Soc Exp Biol Med. 1951 Jan;76(1):38–41. doi: 10.3181/00379727-76-18382. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., BUSCH H. Citric acid content of normal and tumor tissues in vivo following injection of fluoroacetate. Cancer Res. 1950 Jun;10(6):353–356. [PubMed] [Google Scholar]
- Parsons D. F. Recent advances correlating structure and function in mitochondria. Int Rev Exp Pathol. 1965;4:1–54. [PubMed] [Google Scholar]
- Pasquali-Ronchetti I., Greenawalt J. W., Carafoli E. On the nature of the dense matrix granules of normal mitochondria. J Cell Biol. 1969 Feb;40(2):565–568. doi: 10.1083/jcb.40.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A. F., Lowenstein J. M. Citrate content of liver and kidney of rat in various metabolic states and in fluoroacetate poisoning. Biochem J. 1967 May;103(2):342–348. doi: 10.1042/bj1030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner H. B. Studies on the mechanism of shock: the effect of trauma on the toxicity of 3,5 dinitro-o-cresol and sodium fluoroacetate. Br J Exp Pathol. 1969 Jun;50(3):277–284. [PMC free article] [PubMed] [Google Scholar]
- TRUMP B. F., ERICSSON J. L. THE EFFECT OF THE FIXATIVE SOLUTION ON THE ULTRASTRUCTURE OF CELLS AND TISSUES. A COMPARATIVE ANALYSIS WITH PARTICULAR ATTENTION TO THE PROXIMAL CONVOLUTED TUBULE OF THE RAT KIDNEY. Lab Invest. 1965 Jun;14:1245–1323. [PubMed] [Google Scholar]
- Trump B. F., Goldblatt P. J., Stowell R. E. Studies of mouse liver necrosis in vitro. Ultrastructural and cytochemical alterations in hepatic parenchymal cell nuclei. Lab Invest. 1965 Nov;14(11):1969–1999. [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. 3. Effects of iodoacetamide and fluoroacetate on glucose metabolism in the perfused rat heart. J Biol Chem. 1967 Oct 10;242(19):4476–4485. [PubMed] [Google Scholar]
- YANOFF M., ZIMMERMAN L. E., FINE B. S. GLUTARALDEHYDE FIXATION OF WHOLE EYES. Am J Clin Pathol. 1965 Aug;44:167–171. doi: 10.1093/ajcp/44.2.167. [DOI] [PubMed] [Google Scholar]