Abstract
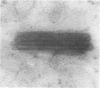
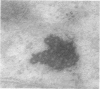
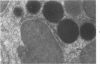
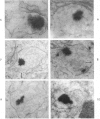
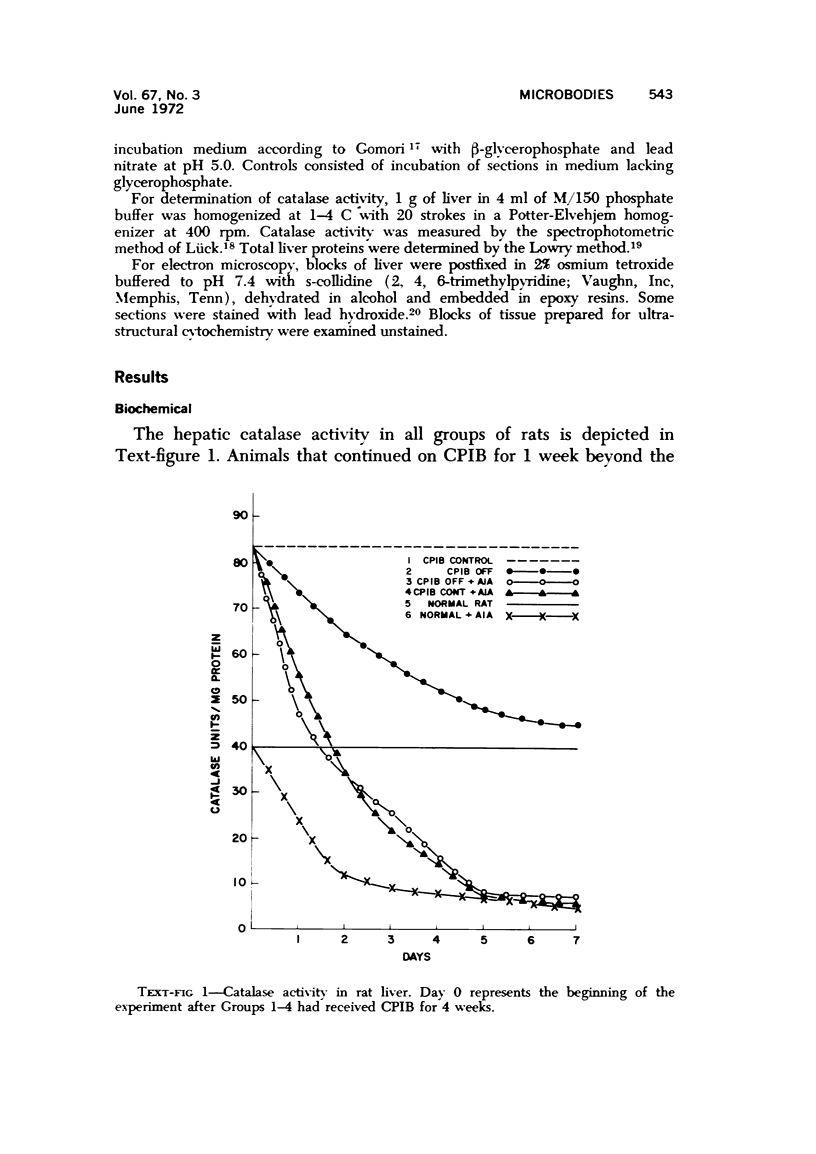
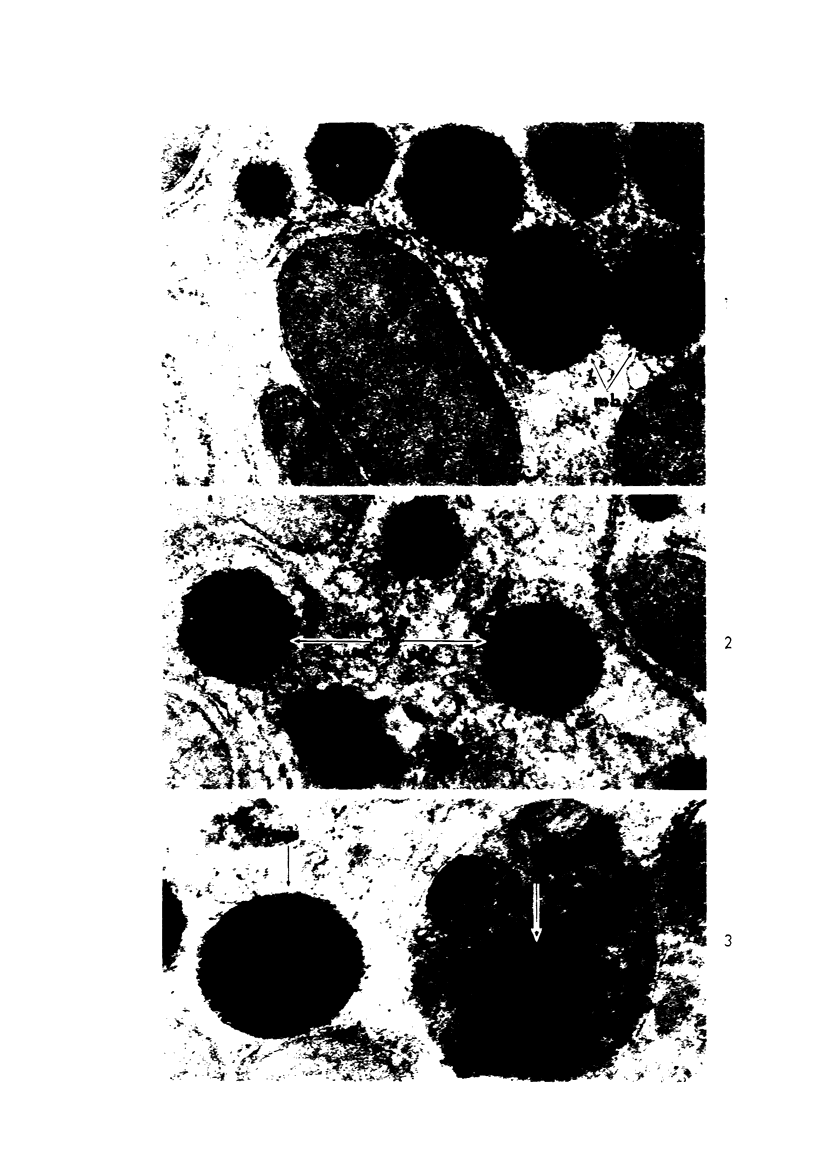
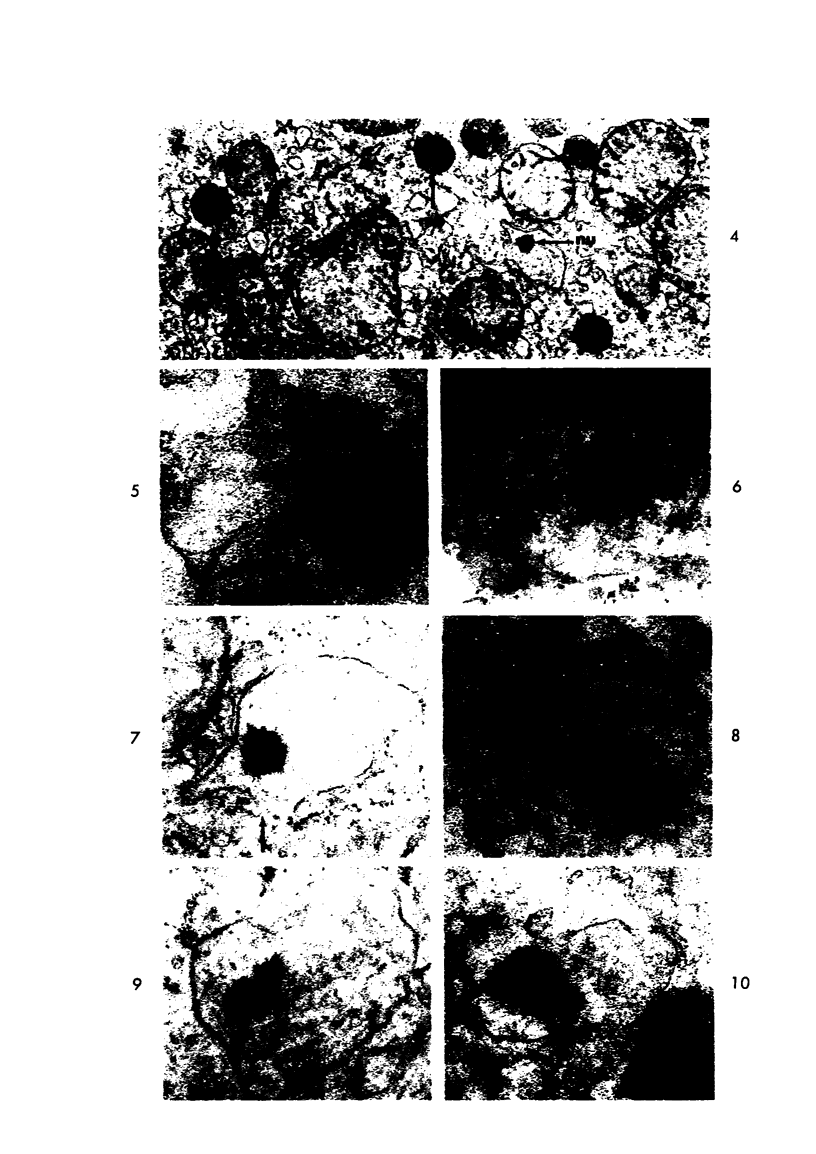
Male rats were given 0.25% CPIB (ethyl chlorophenoxyisobutyrate) for 4 weeks at which time their liver cells showed the typical increase in number of microbodies (peroxisomes). During the first week after withdrawal of CPIB and simultaneous injection of allylisopropylacetamide (AIA), an inhibitor of catalase synthesis, the microbody matrix, in peroxidase preparations, showed marked decrease in electron opacity. In liver cells of rats from which CPIB was withdrawn but which were not given AIA, there were interruption in the limiting membrane and almost total loss of microbody matrix within 3 days. Simultaneous with the loss of matrix content, the hepatic catalase activity decreased from an average 80 units/mg protein to less than 10 units/mg protein. In liver cells of Mastomys natalensis, a multimammate rodent, withdrawal of CPIB was associated with the frequent occurrence of bare microbody nucleoids situated free in the hyaloplasm. The ultrastructural observations and the associated decrease in catalase activity suggest that one means of disposal of microbodies is by rather rapid dissolution or leakage of their matrix enzymes into the surrounding hyaloplasm.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biempica L., Kosower N. S., Nivikoff A. B. Cytochemical and ultrastructural changes in rat liver in experimental porphyria. I. Effects of a single injection of allylisopropylacetamide. Lab Invest. 1967 Aug;17(2):171–189. [PubMed] [Google Scholar]
- Biempica L., Kosower N. S., Roheim P. S. Cytochemical and ultrastructural changes in rat liver in experimental porphyria. II. Effects of repeated injections of allylisopropylacetamide. Lab Invest. 1971 Feb;24(2):110–117. [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Essner E. Endoplasmic reticulum and the origin of microbodies in fetal mouse liver. Lab Invest. 1967 Jul;17(1):71–87. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- HIGASHI T., PETERS T., Jr STUDIES ON RAT LIVER CATALASE. II. INCORPORATION OF 14-C-LEUCINE INTO CATALASE OF LIVER CELL FRACTIONS IN VIVO. J Biol Chem. 1963 Dec;238:3952–3954. [PubMed] [Google Scholar]
- HUDSON P. B., LONDON M. Purification and properties of solubilized uricase. Biochim Biophys Acta. 1956 Aug;21(2):290–298. doi: 10.1016/0006-3002(56)90010-5. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer K. H. Feinstrukturen der Mikrokörper (Microbodies) des proximalen Nierentubulus. Z Zellforsch Mikrosk Anat. 1968;90(3):432–446. [PubMed] [Google Scholar]
- Locke M., McMahon J. T. The origin and fate of microbodies in the fat body of an insect. J Cell Biol. 1971 Jan;48(1):61–78. doi: 10.1083/jcb.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Higashi T., De Duve C. The synthesis and turnover of rat liver peroxisomes. 3. The size distribution of peroxisomes and the incorporation of new catalase. J Cell Biol. 1970 May;45(2):408–415. doi: 10.1083/jcb.45.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Leighton F., De Duve C. The synthesis and turnover of rat liver peroxisomes. II. Turnover of peroxisome proteins. J Cell Biol. 1969 May;41(2):536–546. doi: 10.1083/jcb.41.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J., Bunyaratvej S., Svoboda D. Microbodies in experimentally altered cells. IV. Acatalasemic (CSb) mice treated with CPIB. J Cell Biol. 1969 Aug;42(2):587–596. doi: 10.1083/jcb.42.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J., Chiga M., Bunyaratvej S., Svoboda D. Microbodies in experimentally altered cells. VII. CPID-induced hepatic microbody proliferation in the absence of significant catalase synthesis. J Cell Biol. 1970 Jan;44(1):226–234. doi: 10.1083/jcb.44.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J., Chiga M., Svoboda D. Stimulation of liver catalase synthesis in rats by ethyl-alpha-p-chlorophenoxyisobutyrate. Biochem Biophys Res Commun. 1971 Apr 16;43(2):318–324. doi: 10.1016/0006-291x(71)90755-8. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D. J., Azarnoff D. L. Effects of selected hypolipidemic drugs on cell ultrastructure. Fed Proc. 1971 May-Jun;30(3):841–847. [PubMed] [Google Scholar]
- Svoboda D., Azarnoff D., Reddy J. Microbodies in experimentally altered cells. II. The relationship of microbody proliferation to endocrine glands. J Cell Biol. 1969 Mar;40(3):734–746. doi: 10.1083/jcb.40.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda D., Grady H., Azarnoff D. Microbodies in experimentally altered cells. J Cell Biol. 1967 Oct;35(1):127–152. doi: 10.1083/jcb.35.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]