Abstract
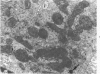
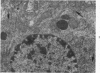
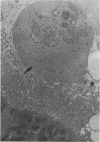
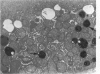
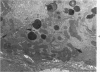
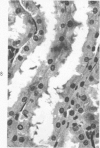
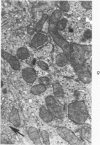
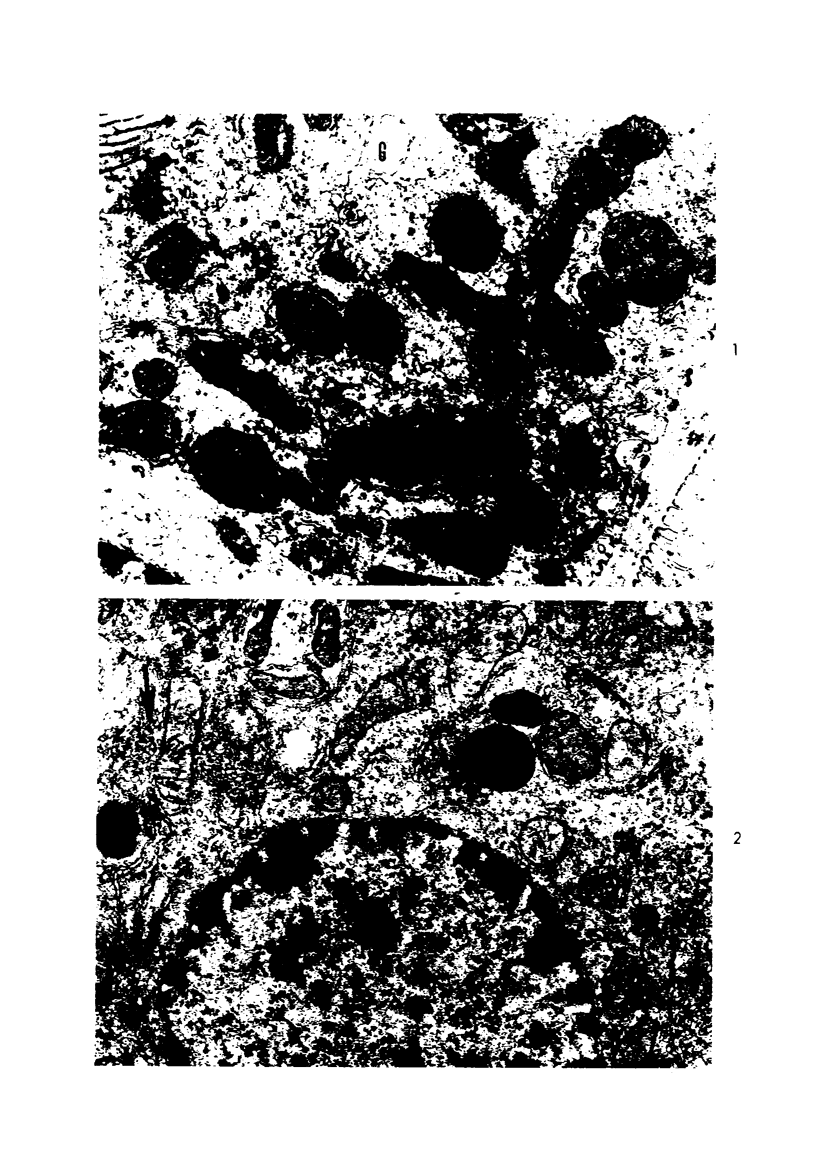
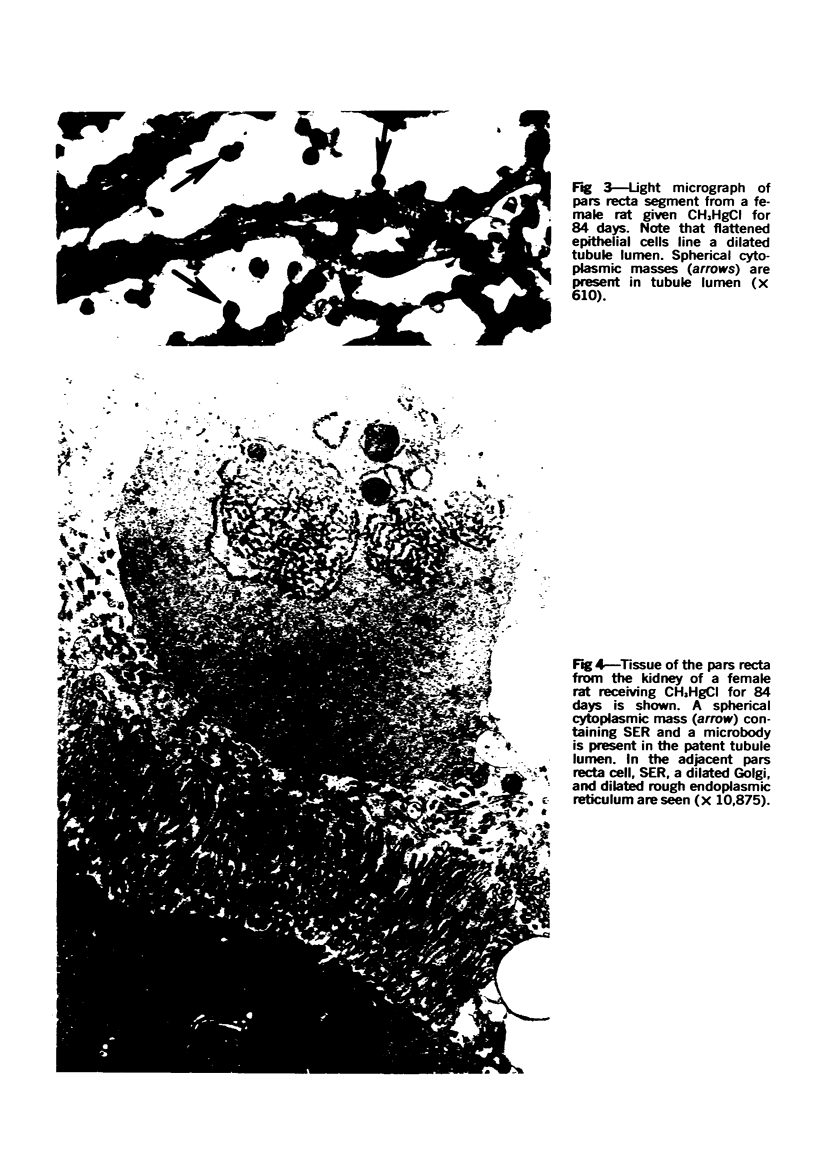
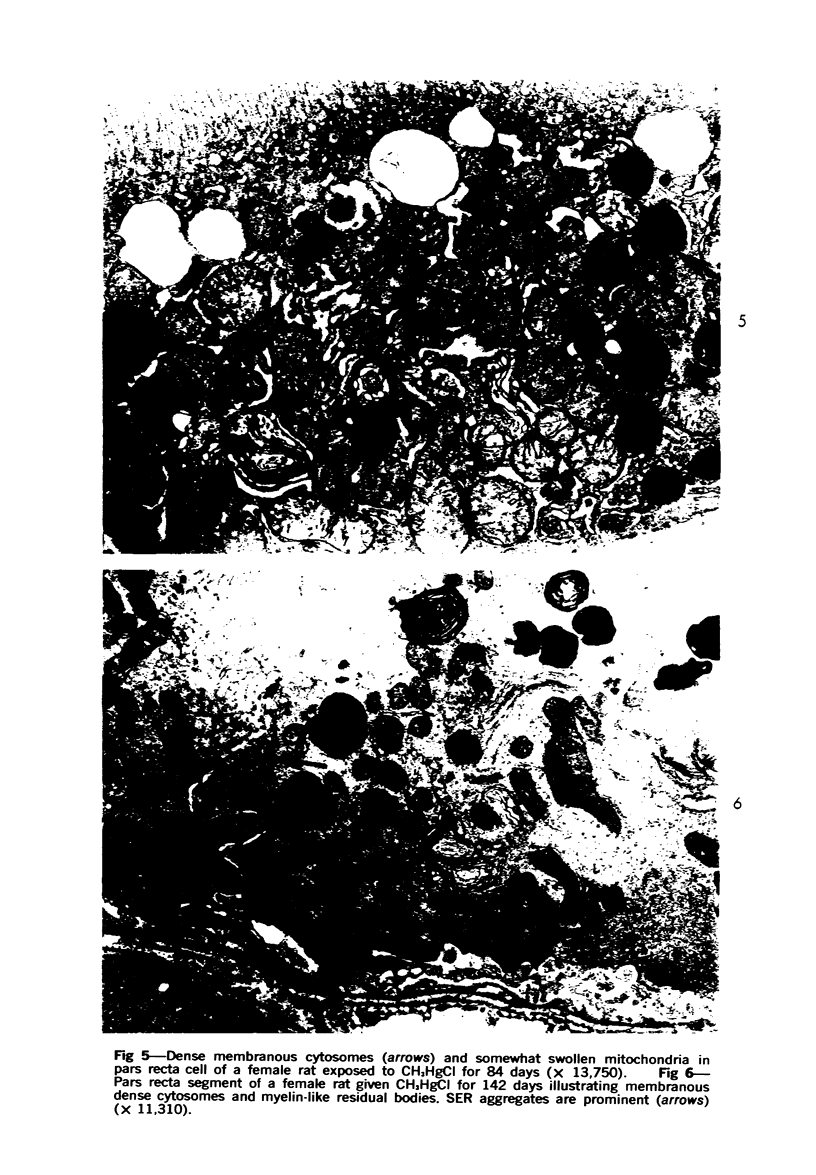
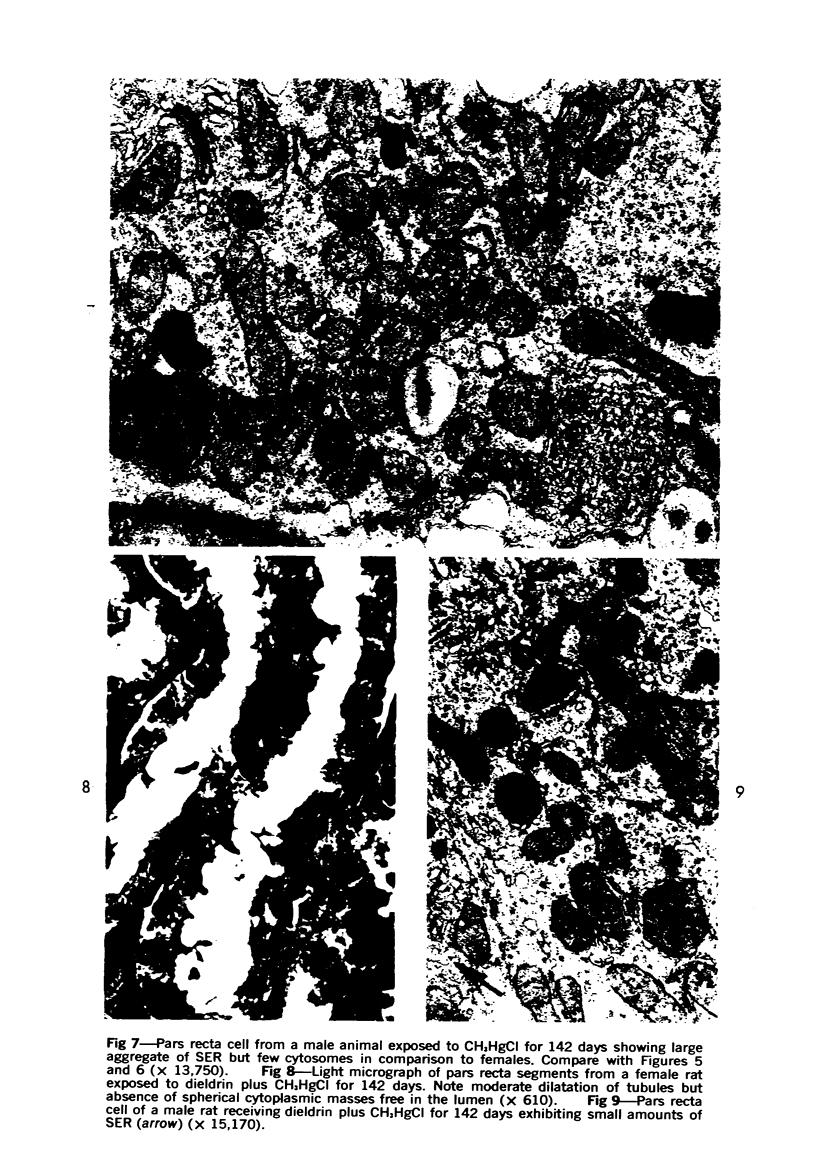
This investigation was undertaken to evaluate the morphologic effects in rat kidney resulting from chronic exposure to low doses of the pesticide dieldrin, methyl mercuric chloride (CH3HgCl) and the combination of dieldrin plus CH3HgCl. Histologic and ultrastructural changes were confined to the proximal tubules. Alterations in these tubules were consistent and reproducible for each regimen and did not become more severe with duration of exposure. The straight segment of the proximal tubule (pars recta) was more severely affected by dieldrin and CH3HgCl than the convoluted portion. Female rats were more markedly affected than males. Pars recta tubule cells of male and female rats exposed to dieldrin showed an increase of smooth endoplasmic reticulum (SER). Male rats displayed a greater increase in SER than females. Pars recta tubule cells of animals given CH3HgCl also exhibited increased amounts of SER, degenerating mitochondria and cell death. Pars recta tubules of females were dilated and contained within the lumens many spherical, hematoxylin-positive staining, cytoplasmic masses, which were visible by light microscopy. These masses were characterized ultrastructurally by the presence of an SER aggregate in an area of material similar to cell matrix. In addition, cells of the pars recta of female animals contained electron-dense membranous cytosomes not present in control animals. Pars recta cells of males showed an increase in SER, but the dense membranous cytosomes observed in the pars recta cells of female rats were not seen. Rats exposed to dieldrin plus CH3CgCl showed less morphologic alteration of the pars recta tubules than animals given methyl mercuric chloride; however, increased amounts of SER and more degeneration in tubule cells were observed in these animals when compared to control animals. The findings are discussed in relation to the conversion of CH3HgCl to inorganic mercury in vivo and the known toxicity of inorganic mercury to the pars recta. Decreased tubular alteration in males and dieldrin-treated animals may be explained by sexual differences in renal enzyme levels or activities and the induction of microsomal enzyme systems by dieldrin.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberg B., Ekman L., Falk R., Greitz U., Persson G., Snihs J. O. Metabolism of methyl mercury (203Hg) compounds in man. Arch Environ Health. 1969 Oct;19(4):478–484. doi: 10.1080/00039896.1969.10666872. [DOI] [PubMed] [Google Scholar]
- BERLIN M. Renal uptake, excretion, and retention of mercury. II. A study in the rabbit during infusion of methyl- and phenylmercuric compounds. Arch Environ Health. 1963 May;6:626–633. [PubMed] [Google Scholar]
- BERLIN M., ULLBERG S. Accumulation and retention of mercury in the mouse. II. An autoradiographic comparison of phenylmercuric acetate with inorganic mercury. Arch Environ Health. 1963 May;6:602–609. doi: 10.1080/00039896.1963.10663448. [DOI] [PubMed] [Google Scholar]
- BERLIN M., ULLBERG S. Accumulation and retention of mercury in the mouse. III. An autoradiographic comparison of methylmercuric dicyandiamide with inorganic mercury. Arch Environ Health. 1963 May;6:610–616. doi: 10.1080/00039896.1963.10663449. [DOI] [PubMed] [Google Scholar]
- Boyd E. M., Chen C. P. Lindane toxicity and protein-deficient diet. Arch Environ Health. 1968 Aug;17(2):156–163. doi: 10.1080/00039896.1968.10665207. [DOI] [PubMed] [Google Scholar]
- Boyd E. M. Dietary protein and pesticide toxicity in male weanling rats. Bull World Health Organ. 1969;40(5):801–805. [PMC free article] [PubMed] [Google Scholar]
- Boyd E. M., Taylor F. I. The acute oral toxicity of chlordane in albino rats fed for 28 days from weaning on a protein-deficient diet. IMS Ind Med Surg. 1969 Dec;38(12):434–441. [PubMed] [Google Scholar]
- Boyd E. M., de Castro E. S. Protein-deficient diet and DDT toxicity. Bull World Health Organ. 1968;38(1):141–150. [PMC free article] [PubMed] [Google Scholar]
- Brooks G. T. The metabolism of diene-organochlorine (cyclodiene) insecticides. Residue Rev. 1969;27:81–138. doi: 10.1007/978-1-4615-8449-0_4. [DOI] [PubMed] [Google Scholar]
- Chedid A., Nair V. Diurnal rhythm in endoplasmic reticulum of rat liver: electron microscopic study. Science. 1972 Jan 14;175(4018):176–179. doi: 10.1126/science.175.4018.176. [DOI] [PubMed] [Google Scholar]
- Eyl T. B., Wilcox K. R., Jr, Reizen M. S. Mercury, fish and human health. Mich Med. 1970 Oct;69(19):873–880. [PubMed] [Google Scholar]
- Fiserova-Bergerova V., Radomski J. L., Davies J. E., Davis J. H. Levels of chlorinated hydrocarbon pesticides in human tissues. Ind Med Surg. 1967 Jan;36(1):65–70. [PubMed] [Google Scholar]
- Fowler B. A., Brooks R. E. Effects of the herbicide paraquat on the ultrastructure of mouse kidney. Am J Pathol. 1971 Jun;63(3):505–520. [PMC free article] [PubMed] [Google Scholar]
- Fowler B. A. Ruthenium red staining of rat glomerulus. Perfusion of ruthenium red into normal and nephrotic rat kidney. Histochemie. 1970;22(2):155–162. doi: 10.1007/BF00303626. [DOI] [PubMed] [Google Scholar]
- Fowler B. A. Ultrastructural evidence for nephropathy induced by long-term exposure tosmall amounts of methyl mercury. Science. 1972 Feb 18;175(4023):780–781. doi: 10.1126/science.175.4023.780. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Szabo E., Koenig H. Isolation and characterization of acidic lipoprotein in renal and hepatic lysosomes. Life Sci II. 1970 Jun 8;9(11):607–616. doi: 10.1016/0024-3205(70)90211-0. [DOI] [PubMed] [Google Scholar]
- Gritzka T. L., Trump B. F. Renal tubular lesions caused by mercuric chloride. Electron microscopic observations: degeneration of the pars recta. Am J Pathol. 1968 Jun;52(6):1225–1277. [PMC free article] [PubMed] [Google Scholar]
- Harr J. R., Claeys R. R., Benedict N. Dieldrin toxicosis in rats: long-term study of brain and vascular effects. Am J Vet Res. 1970 Oct;31(10):1853–1862. [PubMed] [Google Scholar]
- Hutterer F., Klion F. M., Wengraf A., Schaffner F., Popper H. Hepatocellular adaptation and injury. Structural and biochemical changes following dieldrin and methyl butter yellow. Lab Invest. 1969 May;20(5):455–464. [PubMed] [Google Scholar]
- Joselow M. M., Goldwater L. J. Absorption and excretion of mercury in man. XII. Relationship between urinary mercury and proteinuria. Arch Environ Health. 1967 Aug;15(2):155–159. doi: 10.1080/00039896.1967.10664896. [DOI] [PubMed] [Google Scholar]
- KOERNER D. R., HELLMAN L. EFFECT OF THYROXINE ADMINISTRATION ON THE 11-BETA-HYDROXYSTEROID DEHYDROGENASES IN RAT LIVER AND KIDNEY. Endocrinology. 1964 Oct;75:592–601. doi: 10.1210/endo-75-4-592. [DOI] [PubMed] [Google Scholar]
- Kuntzman R., Welch R., Conney A. H. Factors influencing steroid hydroxylases in liver microsomes. Adv Enzyme Regul. 1966;4:149–160. doi: 10.1016/0065-2571(66)90012-4. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res. 1966 Oct;16(3):239–258. doi: 10.1016/s0022-5320(66)80060-6. [DOI] [PubMed] [Google Scholar]
- Morgan D. P., Roan C. C. Chlorinated hydrocarbon pesticide residue in human tissues. Arch Environ Health. 1970 Apr;20(4):452–457. doi: 10.1080/00039896.1970.10665621. [DOI] [PubMed] [Google Scholar]
- Norseth T., Clarkson T. W. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health. 1970 Dec;21(6):717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- Norseth T. The intracellular distribution of mercury in rat liver after methoxyethylmercury intoxication. Biochem Pharmacol. 1967 Sep 9;16(9):1645–1654. doi: 10.1016/0006-2952(67)90239-0. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega P. Light and electron microscopy of dichlorodiphenyltrichloroethane (DDT) poisoning in the rat liver. Lab Invest. 1966 Apr;15(4):657–679. [PubMed] [Google Scholar]
- Ortega P. Partial hepatectomy in rats fed dichlorodiphenyltrichloroethane (DDT). Am J Pathol. 1969 Aug;56(2):229–249. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODIN A. E., CROWSON C. N. Mercury nephrotoxicity in the rat. 1. Factors influencing the localization of the tubular lesions. Am J Pathol. 1962 Sep;41:297–313. [PMC free article] [PubMed] [Google Scholar]
- STREET J. C. DDT ANTAGONISM TO DIELDRIN STORAGE IN ADIPOSE TISSUE OF RATS. Science. 1964 Dec 18;146(3651):1580–1581. doi: 10.1126/science.146.3651.1580. [DOI] [PubMed] [Google Scholar]
- Schiebler T. H., Mühlenfeld E. Uber die geschlechtsspezifische Chemodifferenzierung der Rattenniere. Naturwissenschaften. 1966 Jun;53(12):311–311. doi: 10.1007/BF00712230. [DOI] [PubMed] [Google Scholar]
- Street J. C., Chadwick R. W. Stimulation of dieldrin metabolism by DDT. Toxicol Appl Pharmacol. 1967 Jul;11(1):68–71. doi: 10.1016/0041-008x(67)90027-0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ukita T. Metabolism of ethylmercuric chloride-203Hg in rats. Toxicol Appl Pharmacol. 1970 Jul;17(1):181–188. doi: 10.1016/0041-008x(70)90142-0. [DOI] [PubMed] [Google Scholar]
- Taylor W., Guirgis H. A., Stewart W. K. Investigation of a population exposed to organomercurial seed dressings. Arch Environ Health. 1969 Oct;19(4):505–509. doi: 10.1080/00039896.1969.10666876. [DOI] [PubMed] [Google Scholar]
- Ulfvarson U. The absorption and distribution of mercury in rats fed organs from rats injected with various mercury compounds. Toxicol Appl Pharmacol. 1969 Nov;15(3):525–531. doi: 10.1016/0041-008x(69)90054-4. [DOI] [PubMed] [Google Scholar]
- WEINER I. M., LEVY R. I., MUDGE G. H. Studies on mercurial diureis: renal excretion, acid stability and structure-activity relationships of organic mercurials. J Pharmacol Exp Ther. 1962 Oct;138:96–112. [PubMed] [Google Scholar]
- ZOLLINGER H. U. Cytologic studies with the phase Microscope; the formation of blisters on cells in suspension, photocytosis, with observations on the nature of the cellular membrane. Am J Pathol. 1948 May;24(3):545–567. [PMC free article] [PubMed] [Google Scholar]
- v Deimling O., Baumann G., Noltenius H. Hormonabhängige Enzymverteilung in Geweben. V. Wirkung von Kastration und Sexualhormon auf fünf Enzyme der Mäuseniere. Histochemie. 1965 May 20;5(1):1–10. doi: 10.1007/BF00307886. [DOI] [PubMed] [Google Scholar]