Abstract
Background
Since 1990, the Canadian Red Cross Society and Canadian Blood Services have been testing blood donors for hepatitis C virus (HCV) antibody and HCV nucleic acids and have supplemented HIV antibody testing with p24 antigen testing. We report trends in the incidence of blood-transmissible viral markers and estimates of the risk of undetected infection in donors over the last decade.
Methods
We extracted anonymous donor and blood-transmissible disease information from the Canadian Blood Services National Epidemiology Donor Database for 8.9 million donations from 2.1 million donors between June 1990 and December 2000. The risk of transfusion-transmitted infection (or “residual risk”) refers to the chance that an infected donation escapes detection because of a laboratory test's window period (i.e., the time between infection and detection of the virus by that test). We determined the probability of residual contamination of a unit of blood after testing by using the incidence/window period model, which is based on the incidence of infection in repeat donors and the window period for each laboratory test. The viral markers evaluated in the study were HIV, HCV, hepatitis B virus (HBV) and human T-cell lymphotropic virus (HTLV).
Results
Except for HBV, the transmissible-disease rates of the other evaluated viruses decreased over the study period, with less of a decrease for HTLV. In 2000, the transmissible-disease–positive rate per 100 000 donations was 0.38 for HIV, 16.83 for HCV, 12.40 for HBV and 1.77 for HTLV. The residual risk of HIV, HCV and HTLV decreased over the study period; the residual risk of HBV fluctuated throughout the decade. The current residual risk per million donations is 0.10 for HIV, 0.35 for HCV, 13.88 for HBV and 0.95 for HTLV.
Interpretation
Except for HBV, the estimated risk of undetected infection (residual risk) has decreased over time. The rates of transmissible disease and the probability of undetected transmission of infection are at par with, if not lower than, those reported for other industrialized countries.
Measures to protect the blood supply from the possibility of transfusion-transmitted infection continue to be enhanced. Donor selection criteria and precautionary exclusions have been introduced to protect against clinical and theoretical risks. In addition, improvements in laboratory testing have reduced the risk of transfusion-transmitted infection (Fig. 1).
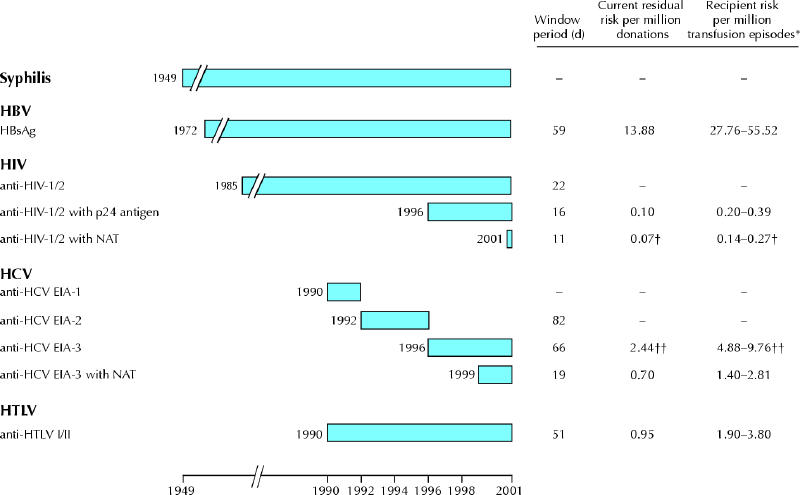
Fig. 1: Timeline showing when laboratory tests for various transmissible diseases were introduced by the Canadian Red Cross Society and Canadian Blood Services. Also shown are corresponding window periods (time between first infection and detection of viral load by the test), residual risks (chance that an infected donation will escape detection because of the test's window period) and risks to recipients undergoing surgical procedures for each test. For hepatitis C virus (HCV), the EIA-1, EIA-2 and EIA-3 tests represent increasingly sensitive new-generation enzyme immunoassays. The tests for syphilis, human T-cell lymphotropic virus (HTLV) and hepatitis B virus (HBV) have not changed over time in terms of sensitivity. Most recently, nucleic acid testing (NAT) for HCV and HIV has been implemented. Note: HBsAg = hepatitis B surface antigen.
*The risk to recipients undergoing surgical procedures (median 2–4 red blood cell units per procedure).1,2,3 For nonsurgical procedures (median 3–5 red blood cell units1), the risk to recipients is derived by multiplying the residual risk by the median number of red blood cell units. The same calculation can be done to determine the risk to recipients per million transfusion episodes of platelets (median 5–8 units4) and fresh frozen plasma (median 4–6 units5). †Estimated based on the incidence rate for 1999–2000 and the HIV NAT window period.6 ††Estimated using the HCV 3 window period and the HCV NAT incidence rate.
With the continued decrease in the incidence of transmissible diseases in the blood supply, it is increasingly difficult to estimate the risk of transfusion-transmitted infection directly. Mathematical models have been developed for this purpose, including the incidence/window period model,7,8,9,10,11,12 which estimates “residual risk” per million donations. Residual risk is the chance that an infected donation will escape detection because of the laboratory test's window period7,8,9 (i.e., the time between first infection and when the viral load becomes detectable by the test). Because the risk in each donated unit equals the risk in each transfused unit, the infection risk to the recipient is directly proportional to the number of unique donor exposures (i.e., transfused units).
Risk estimates based on the incidence/window period model have been reported from many industrialized nations.7,13,14,15,16,17,18,19 In this article, we report trends in detected transmissible diseases between 1990 and 2000 and provide current estimates of residual risk of potentially undetected infection in the blood supply for HIV, hepatitis C virus (HCV), hepatitis B virus (HBV) and human T-cell lymphotropic virus (HTLV). This information is important for determining the safety of blood transfusion and for accurately communicating known risks versus benefits of blood transfusion as a clinical intervention.
Methods
We obtained data from the Canadian Blood Services National Epidemiology Donor Database, which contains Canadian Red Cross Society and Canadian Blood Services records of donors and all donations made by each donor. These donors constitute about 75% of blood donors in Canada; the remainder are clients of Héma-Québec. All personal identifying information was excluded.
The database currently includes records of 13.1 million donations made by 2.4 million blood donors who donated between 1987 and 2001. The data set we used for this study included 8.9 million donations from 2.1 million donors who donated between June 30, 1990, and Dec. 31, 2000. For HCV, the study period began on May 1, 1992, when a second-generation enzyme immunoassay (EIA-2) for HCV was implemented owing to the low sensitivity of the first-generation test (EIA-1). Only records for allogeneic whole blood and apheresis donations were included in the analysis.
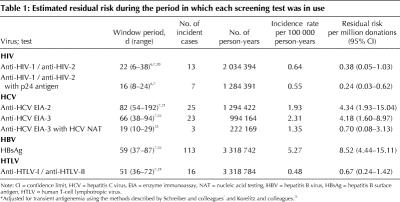
Several generations and versions of laboratory tests were used to screen donations during the study period. We determined window periods for each of these tests using information provided in the test kit inserts and verified them by referring to existing literature on residual risk (Table 1).6,7,20,21,22,23,24 In general, estimates of window periods for HCV and HBV were derived from lookback studies that analyzed information about the time between transfusion-related exposure and the development of a positive test result.9 Estimates of window periods for HIV were derived from mathematical modelling of transfusion-transmitted infections resulting from HIV-seronegative units donated by people who subsequently seroconverted.26
Table 1
For the purpose of analysis, all donors whose supplemental or confirmatory test result following the initial screening test was positive were considered to be positive for transmissible disease.
Transmissible-disease–positive rates, per 100 000 donations, were calculated for the 4 viral markers as the number of donors who tested positive divided by the total number of donations. Rates were calculated separately for donations from first-time donors and for donations from repeat donors, who presumably were free of infection at the time of their previous donation. Incidence rates, per 100 000 person-years, were calculated using only those repeat donors who made at least 2 donations within 3 years during the study period. For all disease-negative donors, person-years were calculated as the sum of all interdonation intervals of 3 years or less within the study period. For confirmed positive donors, person-years were calculated as the sum of all interdonation intervals of 3 years or less within the study period up to the midpoint of the negative–positive interdonation interval. This assumes that seroconversion occurred at the midpoint between the last negative donation and the positive donation. Linear trends in transmissible-disease–positive rates and incidence rates over time were tested using Poisson regression methods.
Approximate 95% confidence intervals (CIs) were calculated for all incidence rates assuming that the number of donors who tested positive followed a Poisson distribution, with rate proportional to the total period of observation (i.e., the total person-years at risk). When the number of incident cases was greater than 5, the CI was based on a logarithmic transformation, as shown in the following equation:
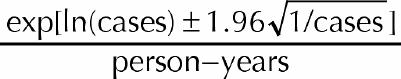 |
When the number of incident cases was fewer than 5, the exact CI was calculated using the lower and upper limits as stated in Rosner27 divided by total person-years.
The residual risk of transfusion-transmitted infection per million donations was calculated for each viral marker as the product of the incidence rate and the length of the window period (in years). An approximate 95% CI for the residual risk was calculated by multiplying the end points of the CI for the incidence rate by the end points of the window period.7
Finally, because HBV infection may not always be detected by hepatitis B surface antigen (HBsAg), we adjusted all HBV incidence rates to account for this “transient antigenemia” using a method described by Schreiber and colleagues7 and Korelitz and colleagues.25 Adjusted incidence rates were calculated by multiplying the crude rate by 1/[0.05 + (0.70 х T)], where T, the probability of detecting HBsAg, is estimated by dividing the duration of transient antigenemia (63 days) by the observed median interval between donations for all HBV incident cases.
Results
Disease-positive rates
In 2000, Canadian Blood Services collected 790 460 whole-blood and apheresis donations. Of these, 3 were confirmed positive for HIV, 133 positive for HCV, 98 positive for HBV and 14 positive for HTLV. The transmissible-disease–positive rates per 100 000 were 0.38 for HIV, 16.83 for HCV, 12.40 for HBV and 1.77 for HTLV. Fig. 2 shows the trends in disease-positive rates from 1990 to 2000 by donation status (first time or repeat). Except for HIV in 1999–2000, the disease-positive rates were several times greater among the first-time donations than among the repeat donations.
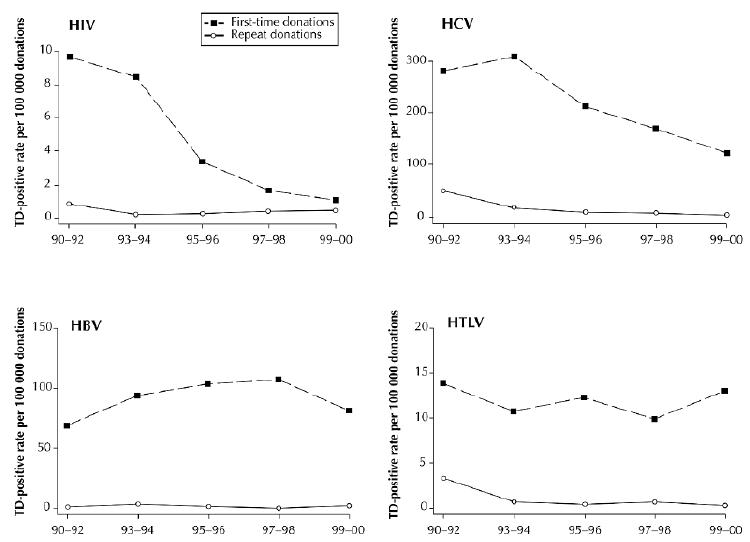
Fig. 2: Incidence rates of HIV, HCV, HBV and HTLV markers per 100 000 first-time and 100 000 repeat donations, 1990–2000. TD = transmissible disease. Photo: Chesley Sheppard
Among the first-time donations, HIV-positive rates decreased significantly, from 9.6 per 100 000 in 1990 to 1.0 per 100 000 in 2000 (p = 0.008). In 1992, a new screening test for HCV was introduced, accounting for the increased HCV-positive rate observed between 1990 and 1993. By 2000, the HCV-positive rate had decreased significantly to 120.6 per 100 000 (p = 0.029). Unlike the HIV- and HCV-positive rates, no significant linear decrease was observed in the HBV- and HTLV-positive rates (p = 0.39 and p = 0.52 respectively). HBV-positive rates peaked at 108.0 per 100 000 in 1997–1998 and declined thereafter.
Among the repeat donations, the HIV- and HBV-positive rates remained low and relatively stable throughout the study period (test for linear trend, p = 0.39 and p = 0.75 respectively). In 1999–2000, the disease-positive rates were 0.43 per 100 000 for HIV and 2.10 per 100 000 for HBV. However, significant declines were observed in the HCV- and HTLV-positive rates (p = 0.002 and p = 0.034 respectively). Much of the decline occurred by 1995–1996 for HCV and by 1993–1994 for HTLV. In 1999–2000, the disease-positive rates were 2.46 per 100 000 for HCV and 0.29 per 100 000 for HTLV.
Incidence and current residual risk
Estimates of residual risk for each screening test were based on the entire period during which the test was in use (Table 1). With the introduction of HIV p24 antigen testing, the residual risk of HIV decreased from 0.38 per million donations to 0.24 per million. When anti-HCV EIA-3 testing was introduced, the residual risk of HCV decreased from 4.34 per million to 4.18 per million. A greater reduction was observed when HCV nucleic acid testing (NAT) was introduced: with both the anti-HCV EIA-3 and the HCV NAT, the risk was 0.70 per million, or a sixth of the risk during EIA-2 testing. Risk estimates for HBV and HTLV are based on the entire 10-year study period because the screening tests did not change during this time.
When we examined the data by 2-year intervals over the study period, we found that the residual risk of HIV infection decreased from 1.43 per million donations in 1990–1992 to 0.10 per million in 1999–2000 (a table showing the incidence rates and residual risk of transfusion-transmitted infection by 2-year intervals is available with the online version of the article [www.cmaj.ca]). Overall, the residual risk of HCV infection also declined over time, although an increased risk was observed in 1995–1996, which coincided with the introduction of EIA-3 testing for HCV; enhanced detection may have been responsible for this increase. There were 21 cases of HCV in 1995–1996: 7 occurred in 1995 and 14 in 1996. Thus, the increase in residual risk during this period is attributable to an increase in the incidence of HCV. For the last 2-year period (1999–2000) only, the estimate for HCV is 0.35 per million (CI 0.04–1.57), as indicated in the online web table. As a result of the changing incidence of HBV and a constant window period, the residual risk of HBV fluctuated throughout the study period, peaking during 1993–1994 and 1999–2000 at 13.93 and 13.88 per million donations respectively. For HTLV, the very small number of cases overall suggests that the current risk of HTLV in the blood supply can be no greater than the upper limit of the 1999–2000 CI of 1.64 per million. Because there were no HTLV incident cases in 1999–2000, the estimate of 0.95 per million donations in 1997–1998 is used to estimate current risk.
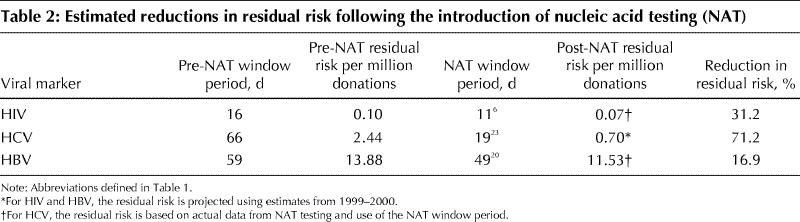
The introduction of NAT has had an effect on residual risk and risk reduction (Table 2). Because NAT for HIV was introduced only in 2001, we did not estimate its effect on the residual risk of transmitting HIV in this study. Instead, we projected the residual risk by using data from current tests and the HIV NAT window period. Results indicate that the recent availability of NAT could reduce the residual risk of HIV and HCV infection by about 30% and 70% respectively. Based on a prediction for HBV NAT, with a window period of 49 days,20 the residual risk would be reduced from 13.88 per million donations to 11.53 per million.
Table 2
Undetected infection in first-time donors
Although unknown, the rate of undetected infection among first-time donors has been estimated to be about twice the rate among repeat donors.13,20,28 Because first-time donors accounted for 13% of all donations during the study period, the overall incidence of HIV and HCV during 1999–2000 may have been as high as 0.25 and 0.76 per 100 000 person-years respectively (i.e., overall incidence = [0.13 х 2 х incidence rate among repeat donors] + [0.87 х incidence rate among repeat donors]). Therefore, the residual risks of HIV and HCV during 1999–2000 may have increased from 0.10 to 0.11 per million donations and from 0.35 to 0.40 per million respectively.
Risk to transfusion recipients
Because the residual risk of transmissible infection in each donated unit equals the risk in each transfused unit, the risk of infection increases with the number of units transfused. About 6% of all inpatients receive at least 1 unit of blood.1 Red blood cells constitute at least 95% of all transfusions given.29 Examples of the possible increase in risk to recipients by the median number of units of red blood cells given are listed in Fig. 1. These extrapolations do not apply to fractionated products, which are heat or detergent treated, or to recombinant coagulation factor products, which do not pose a risk of transfusion-transmitted infection.
Interpretation
Over the last decade, although HBV rates have remained relatively constant, disease-positive rates, incidence rates and residual risks of HIV and HCV have decreased, and after an initial reduction HTLV rates have remained low and unchanged. The decrease in HIV- and HCV-positive rates suggests that donor education and screening may have been effective.
The incidence/window period model assumes infectivity of the virus throughout the window period, an assumption that, if invalid, could result in an overestimate of risk of transfusion-transmitted infection. On the other hand, the risk could be underestimated because of possibly undetected viral variants, immunosilent infections or laboratory error.
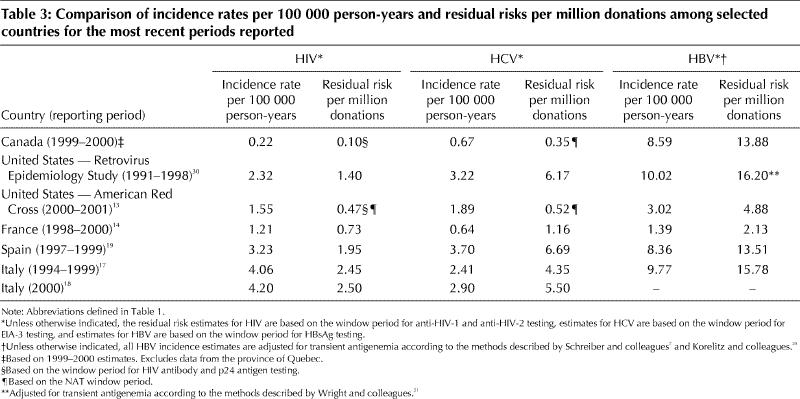
We compared our residual risk estimates with estimates from other countries that used the same incidence/window period model (Table 3). Our 1999–2000 risk estimate for HIV using the window period for p24 antigen testing is less than 0.10 per million donations; the American Red Cross, the only other blood service using p24 antigen testing, found a residual risk of 0.47 per million donations. Our HCV risk estimate of 0.35 per million donations, with HCV NAT in place, is lower than the American Red Cross risk estimate of 0.52 per million. The elevated rates in the United States may be a result of greater prevalence of some transmissible diseases. For example, according to a United Nations report,32 the proportion of people with HIV/AIDS in the United States is twice that in Canada (0.6% v. 0.3%). The difference in residual risk between the 2 countries could also be attributed to the role of universal health care coverage in Canada, which allows ready access to testing for transmissible diseases. Our adjusted HBV risk of 13.88 per million donations is similar to other adjusted estimates, except for those of the American Red Cross (4.88 per million) and those from France (2.13 per million). Our estimate of the residual risk of HTLV of 0.95 per million donations falls between those of the American Red Cross and France for a similar period.13,14
Table 3
Is our blood supply as safe as it can be? We conclude that it is as safe as state-of-the-art methods in industrialized countries allow. However, we cannot say that a zero-risk blood supply has been achieved here or elsewhere. The current risk of transfusion-transmitted infection attributable to repeat donors is extremely low, with an estimated per-unit risk of 1 in 10 million for HIV, 1 in 3 million for HCV, 1 in 72 000 for HBV and 1 in 1.1 million for HTLV. Despite advances in testing, it remains critically important to maintain a rigorous donor selection process. Appropriately focused donor education regarding inclusion and exclusion criteria together with state-of-the-art testing have brought us to the current level of safety.
Acknowledgments
We acknowledge the excellent assistance of Vito Scalia, Edna Zuber and the staff of the Canadian Blood Services (CBS) National Testing Laboratory. In addition, over the last several years, Steve Brulé and his staff in the Information Services Division of CBS have facilitated the establishment of the National Epidemiology Donor Database, without which we could not have carried out transmissible disease and donor surveillance, nor produce reports, such as the current one, on the status of our blood supply.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Chiavetta was responsible for the development and implementation of the surveillance system; she was chief investigator in the study and primary author of this report. Dr. Escobar consulted on and supervised the statistical analysis and presentation of the data. Ms. Newman was responsible for programming and the development of the surveillance database and assisted in the preparation of the manuscript. Dr. He assisted with the statistical analysis and the drafting of the manuscript. Mr. Driezen had a key role in writing the manuscript and presenting the data. Dr. Deeks supervised the development and validation of the surveillance database and participated in the development of the statistical models. Mr. Hone helped compose and edit the manuscript. Dr. O'Brien assisted with the consolidation and revision of the last draft of the manuscript. Dr. Sher assisted in the development of the surveillance database and the interpretation of the data.
Competing interests: None declared.
Correspondence to: Dr. Jo Anne Chiavetta, Epi-Stat Research Inc., 910–44 Charles St. W, Toronto ON M4Y 1R7; jchiavetta@sympatico.ca
References
- 1.Chiavetta JA, Herst R, Freedman J, Axcell TJ, Wall AJ, van Rooy SC. A survey of red cell use in 45 hospitals in central Ontario, Canada. Transfusion 1996; 36:699-706. [DOI] [PubMed]
- 2.Pinkerton PH, Tasev T, Coovadia AS. Changes in red-cell transfusion practice in a tertiary care hospital during the 1990s — a 7-year study. Transfus Med 1998;8:179-84. [DOI] [PubMed]
- 3.Sirchia G, Giovanetti AM, McClelland DBL, Fracchia GN, editors. Safe and good use of blood in surgery (SANGUIS) — use of blood products and artificial colloids in 43 European hospitals. Luxembourg: Office for Official Publications of the European Communities; 1994.
- 4.Clinical guide to transfusion. 3rd ed. Ottawa: Canadian Red Cross Society; 1993.
- 5.Zimmermann R, Büscher M, Linhardt C, Handtrack D, Zingsem J, Weisbach V, et al. A survey of blood component use in a German university hospital. Transfusion 1997;37:1075-83. [DOI] [PubMed]
- 6.Busch MP, Lee LLL, Satten GA, Henrard DR, Farzadegan H, Nelson KE, et al. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion 1995;35:91-7. [DOI] [PubMed]
- 7.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med 1996;334:1685-90. [DOI] [PubMed]
- 8.Kleinman S, Busch MP, Korelitz JJ, Schreiber GB. The incidence/window period model and its use to assess the risk of transfusion-transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev 1997; 11:155-72. [DOI] [PubMed]
- 9.Kleinman SH, Busch MP. The risks of transfusion-transmitted infection: direct estimation and mathematical modelling. Baillieres Best Pract Res Clin Haematol 2000;13:631-49. [DOI] [PubMed]
- 10.Müller-Breitkreutz K. Results of viral marker screening of unpaid blood donations and probability of window period donations in 1997. EPFA Working Group of Quality Assurance. Vox Sang 2000;78:149-57. [DOI] [PubMed]
- 11.Correll PK, Law MG, Seed CR, Gust A, Buring M, Dax EM, et al. Variant Creutzfeld–Jakob disease in Australian blood donors: estimation of risk and the impact of deferral strategies. Vox Sang 2001;81:6-11. [DOI] [PubMed]
- 12.Seed CR, Cheng A, Ismay SL, Bolton WV, Kiely P, Cobain TJ, et al. Assessing the accuracy of three viral risk models in predicting the outcome of implementing HIV and HCV NAT donor screening in Australia and the implications for future HBV NAT. Transfusion 2002;42:1365-72. [DOI] [PubMed]
- 13.Dodd RY, Notari EP IV, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion 2002;42:975-9. [DOI] [PubMed]
- 14.Pillonel J, Laperche S, Saura C, Desenclos JC, Couroucé AM. Trends in residual risk of transfusion-transmitted viral infections in France between 1992 and 2000. Transfusion 2002;42:980-8. [DOI] [PubMed]
- 15.Schwartz DW, Simson G, Baumgarten K, Fabritz H, Riggert J, Neumeyer H, et al. Risk of human immunodeficiency virus (HIV) transmission by anti-HIV-negative blood components in Germany and Austria. Ann Hematol 1995; 70: 209-13. [DOI] [PubMed]
- 16.Riggert J, Schwartz DWM, Uy A, Simson G, Jelinek F, Fabritz H, et al. Risk of hepatitis C virus (HCV) transmission by anti-HCV-negative blood components in Austria and Germany. Ann Hematol 1996;72:35-9. [DOI] [PubMed]
- 17.Tosti ME, Solinas S, Prati D, Salvaneschi L, Manca M, Francesconi M, et al. An estimate of the current risk of transmitting blood-borne infections through blood transfusion in Italy. Br J Haematol 2002;117:215-9. [DOI] [PubMed]
- 18.Velati C, Romano L, Baruffi L, Pappalettera M, Carreri V, Zanetti AR. Residual risk of transfusion-transmitted HCV and HIV infections by antibody-screened blood in Italy. Transfusion 2002;42:989-93. [DOI] [PubMed]
- 19.Alvarez M, Oyonarte S, RodrÍguez PM, Hernández JM. Estimated risk of transfusion-transmitted viral infections in Spain. Transfusion 2002;42:994-8. [DOI] [PubMed]
- 20.Glynn SA, Kleinman SH, Wright DJ, Busch MP. International application of the incidence rate/window period model. Transfusion 2002;42:966-72. [DOI] [PubMed]
- 21.Busch MP, Korelitz JJ, Kleinman SH, Lee SR, AuBuchon JP, Schreiber GB. Declining value of alanine aminotransferase in screening of blood donors to prevent posttransfusion hepatitis B and C virus infection. The Retrovirus Epidemiology Donor Study. Transfusion 1995;35:903-10. [DOI] [PubMed]
- 22.Mimms LT, Mosley JW, Hollinger FB, Aach RD, Stevens CE, Cunningham M, et al. Effect of concurrent acute infection with hepatitis C virus on acute hepatitis B virus infection. BMJ 1993;307:1095-7. [DOI] [PMC free article] [PubMed]
- 23.Flanagan P, Barbara J. PCR testing of plasma pools: from concept to reality. Tranfus Med Rev 1999;13:164-76. [DOI] [PubMed]
- 24.Manns A, Wilks RJ, Murphy EL, Haynes G, Figueroa JP, Barnett M, et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer 1992;51:886-91. [DOI] [PubMed]
- 25.Korelitz JJ, Busch MP, Kleinman SH, Williams AE, Gilcher RO, Ownby HE, et al. A method for estimating hepatitis B virus incidence rates in volunteer blood donors. National Heart, Lung and Blood Institute Retrovirus Epidemiology Donor Study. Transfusion 1997;37:634-40. [DOI] [PubMed]
- 26.Busch MP. Closing the windows on viral transmission by blood transfusion. In: Stramer SL, editor. Blood safety in the new millenium. Bethesda: American Association of Blood Banks; 2001. p. 45.
- 27.Rosner B. Confidence limits for the expectation of a Poisson variable. Table 8. In: Fundamentals of biostatistics. 4th. ed. Toronto: Duxbury Press; 1995. p. 653.
- 28.Janssen RS, Satten GA, Stramer SL, Rawal BD, O'Brien TR, Weiblen BJ, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 1998;280:42-8. [DOI] [PubMed]
- 29.Chiavetta JA, Herst R, Freedman J, Axcell T, van Rooy S. Red cell transfusion patterns in 45 hospitals. In Proceedings from the Joint Scientific Conference: final program, 23–26 May 1996. Toronto: Canadian Society for Transfusion Medicine/Canadian Red Cross Society; 1996. p. 22.
- 30.Glynn S. Residual Risks for hepatitis and HIV and unreported deferrable risks in US blood donors. In: Chiavetta JA, Deeks S, Goldman M, Hannon J, Leach-Bennett J, Megânn H, et al. Proceedings of a consensus conference: blood-borne HIV and hepatitis — optimizing the donor selection process. Transfus Med Rev 2003;17:1–30. [DOI] [PubMed]
- 31.Wright DJ, Glynn SA, Busch M, Kleinman S, Watanabe KK, Schreiber GB. A proposed new method to estimate hepatitis B incidence rates based on HBsAg marker. Transfusion 1999;39(Suppl):106S.
- 32.Report on the global HIV/AIDS epidemic 2002. Geneva: Joint United Nations Programme on HIV/AIDS; 2002. Available: www.unaids.org/en/resources/epidemiology.asp (accessed 2003 Sept 9).