Abstract
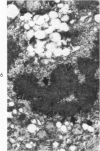
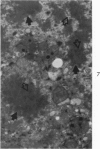
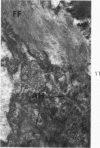
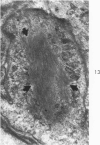
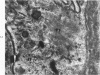
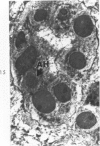
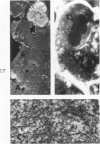
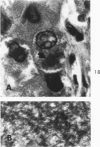
Liver biopsies obtained from 24 patients with alcoholic liver disease were studied by light and electron microscopy. Comparisons of the same cells in adjacent sections revealed that alcoholic hyalin is a fibrillar deposit without limiting membranes and is readily distinguished from giant mitochondria. This characteristic fibrillar structure was encountered in hepatocytes, ductular cells and in benign and malignant hepatomas. Three distinct morphologic forms of alcoholic hyalin were observed: a) bundles of filaments in parallel arrays, b) clusters of randomly oriented fibrils and c) a granular or amorphous substance containing only scattered remains of fibrils. Closely associated with alcoholic hyalin and often found along its entire circumference, were bundles of fine filaments in parallel arrangement of much smaller size. These occasionally displayed variations in orientation and merged with the filaments in the alcoholic hyalin body. Similar fine filaments were observed, in considerable excess, in cells which did not contain alcoholic hyalin. According to our findings, the fine filaments and the significantly larger filaments in alcoholic hyalin could be parts of a contractile system elaborated by host cells during the course of hepatic injury.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRATI A., Jr Submicroscopic structure of Yoshida ascites hepatoma. Cancer Res. 1961 Sep;21:989–992. [PubMed] [Google Scholar]
- BIAVA C. G. STUDIES ON CHOLESTASIS. A RE-EVALUATION OF THE FINE STRUCTURE OF NORMAL HUMAN BILE CANALICULI. Lab Invest. 1964 Aug;13:840–864. [PubMed] [Google Scholar]
- BIAVA C. MALLORY ALCOHOLIC HYALIN: A HERETOFORE UNIQUE LESION OF HEPATOCELLULAR ERGASTOPLASM. Lab Invest. 1964 Apr;13:301–320. [PubMed] [Google Scholar]
- Biberfeld P., Ericsson J. L., Perlmann P., Raftell M. Increased occurrence of cytoplasmic filaments in in vitro propagated rat liver epithelial cells. Exp Cell Res. 1965 Aug;39(1):301–305. doi: 10.1016/0014-4827(65)90034-0. [DOI] [PubMed] [Google Scholar]
- EDMONDSON H. A., PETERS R. L., REYNOLDS T. B., KUZMA O. T. SCLEROSING HYALINE NECROSIS OF THE LIVER IN THE CHRONIC ALCOHOLIC. A RECOGNIZABLE CLINICAL SYNDROME. Ann Intern Med. 1963 Nov;59:646–673. doi: 10.7326/0003-4819-59-5-646. [DOI] [PubMed] [Google Scholar]
- FLAX M. H., TISDALE W. A. AN ELECTRON MICROSCOPIC STUDY OF ALCOHOLIC HYALIN. Am J Pathol. 1964 Mar;44:441–453. [PMC free article] [PubMed] [Google Scholar]
- Iseri O. A., Gottlieb L. S. Alcoholic hyalin and megamitochondria as separate and distinct entities in liver disease associated with alcoholism. Gastroenterology. 1971 Jun;60(6):1027–1035. [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak N. C., Sagreiya K., Ramalingaswami V. Indian childhood cirrhosis. The nature and significance of cytoplasmic hyaline of hepatocytes. Arch Pathol. 1969 Dec;88(6):631–637. [PubMed] [Google Scholar]
- Newstead J. D. Filaments in renal parenchymal and interstitial cells. J Ultrastruct Res. 1971 Feb;34(3):316–328. doi: 10.1016/s0022-5320(71)80075-8. [DOI] [PubMed] [Google Scholar]
- Norkin S. A., Campagna-Pinto D. Cytoplasmic hyaline inclusions in hepatoma. Histochemical study. Arch Pathol. 1968 Jul;86(1):25–32. [PubMed] [Google Scholar]
- PORTA E. A., BERGMAN B. J., STEIN A. A. ACUTE ALCOHOLIC HEPATITIS. Am J Pathol. 1965 Apr;46:657–689. [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Ito S. Cytoplasmic filaments of Amoeba proteus. I. The role of filaments in consistency changes and movement. J Cell Biol. 1970 Aug;46(2):267–289. doi: 10.1083/jcb.46.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer-Danneel S. Strukturelle und funktionelle Voraussetzungen für die Bewegung von Amoeba proteus. Z Zellforsch Mikrosk Anat. 1967;78(4):441–462. [PubMed] [Google Scholar]
- Smuckler E. A. The ultrastructure of human alcoholic hyalin. Am J Clin Pathol. 1968 Jun;49(6):790–797. doi: 10.1093/ajcp/49.6.790. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]