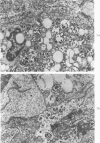
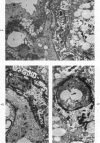
Abstract
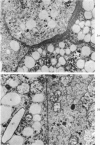
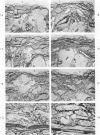
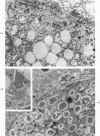
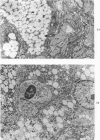
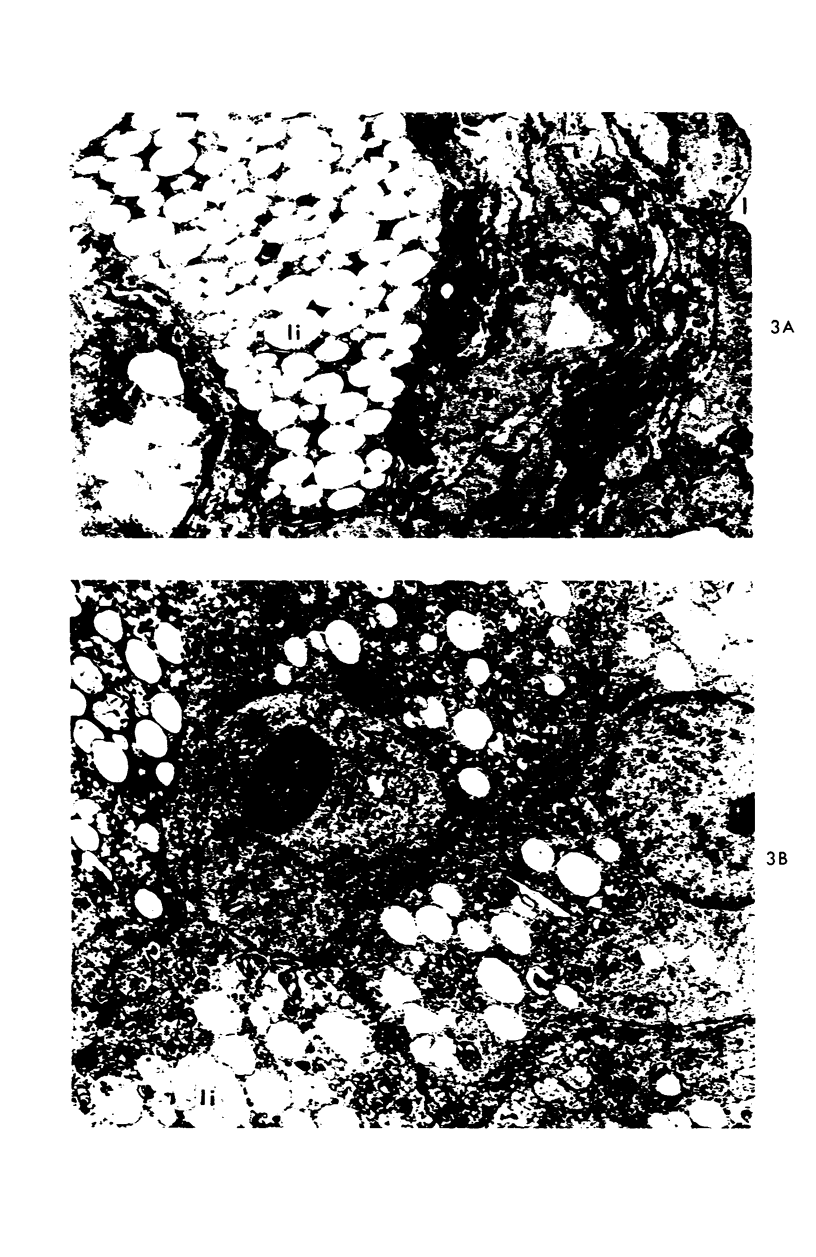
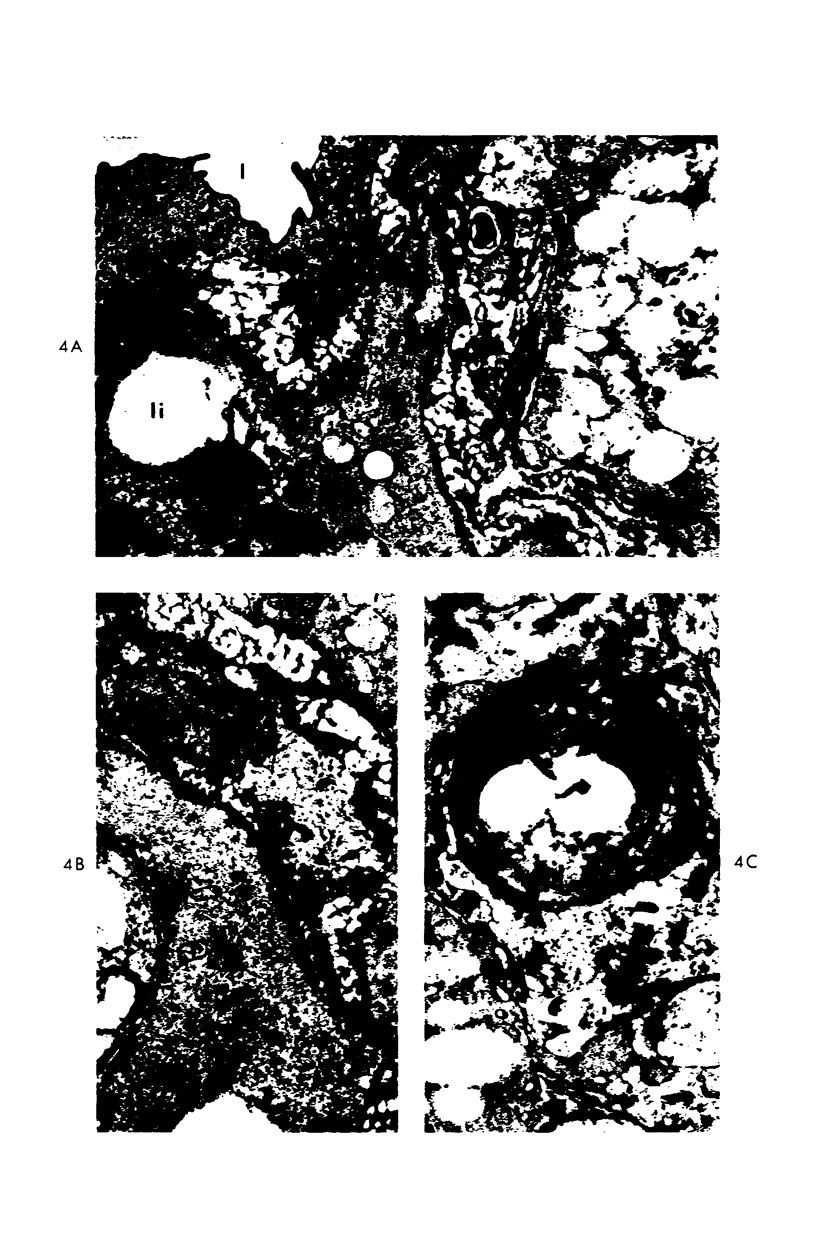
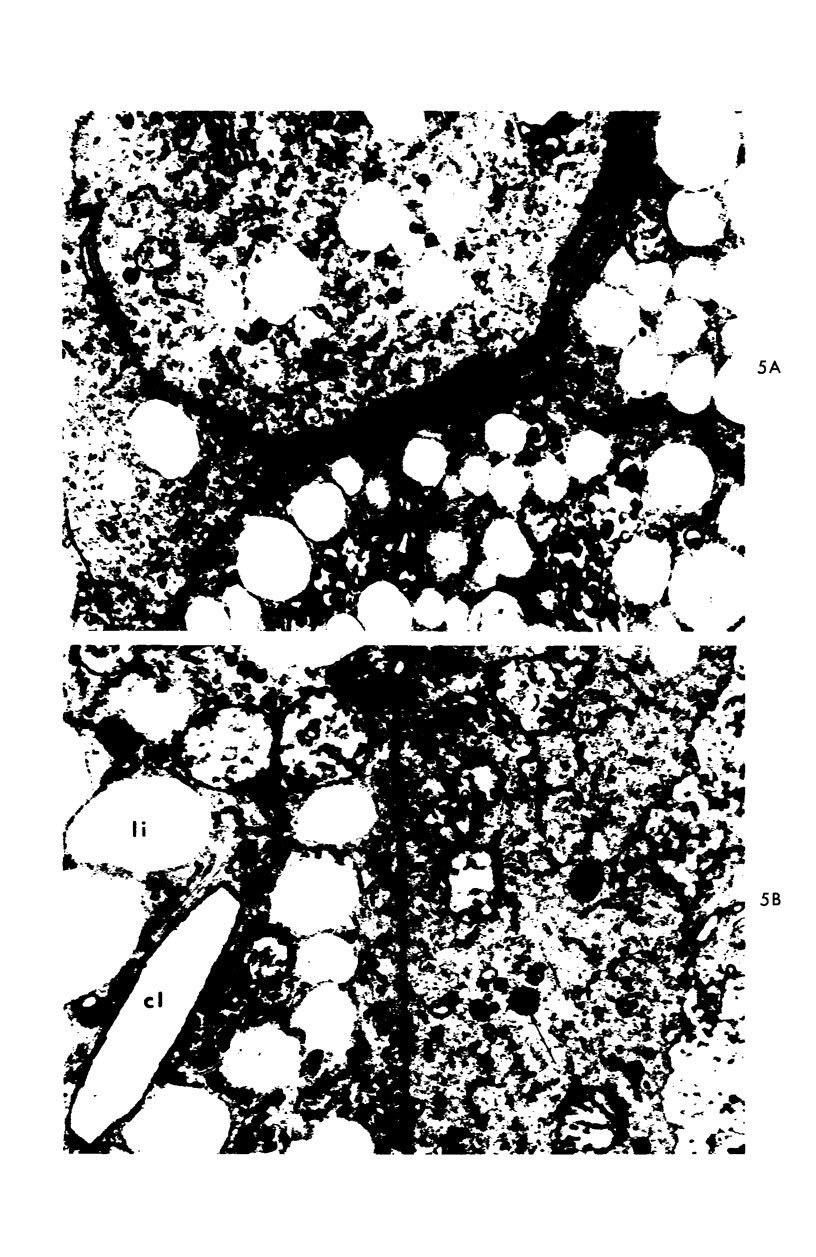
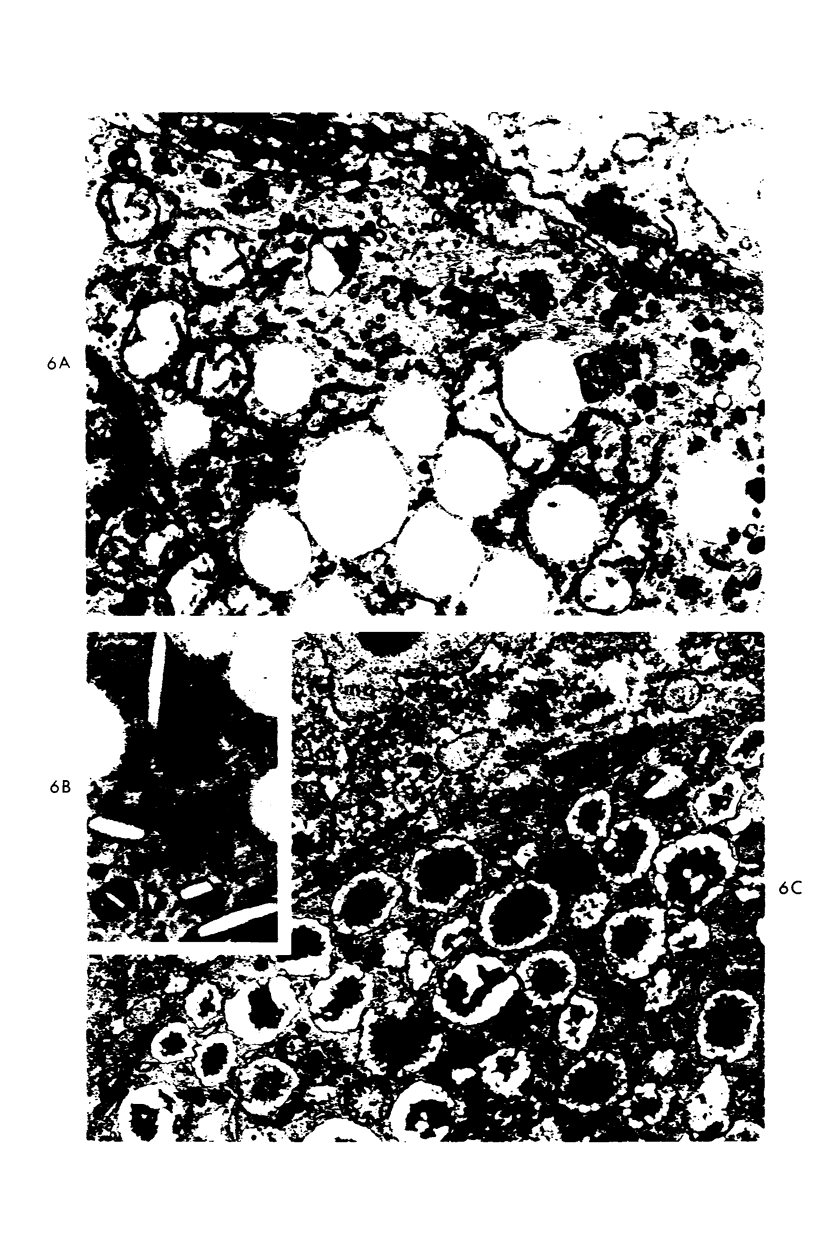
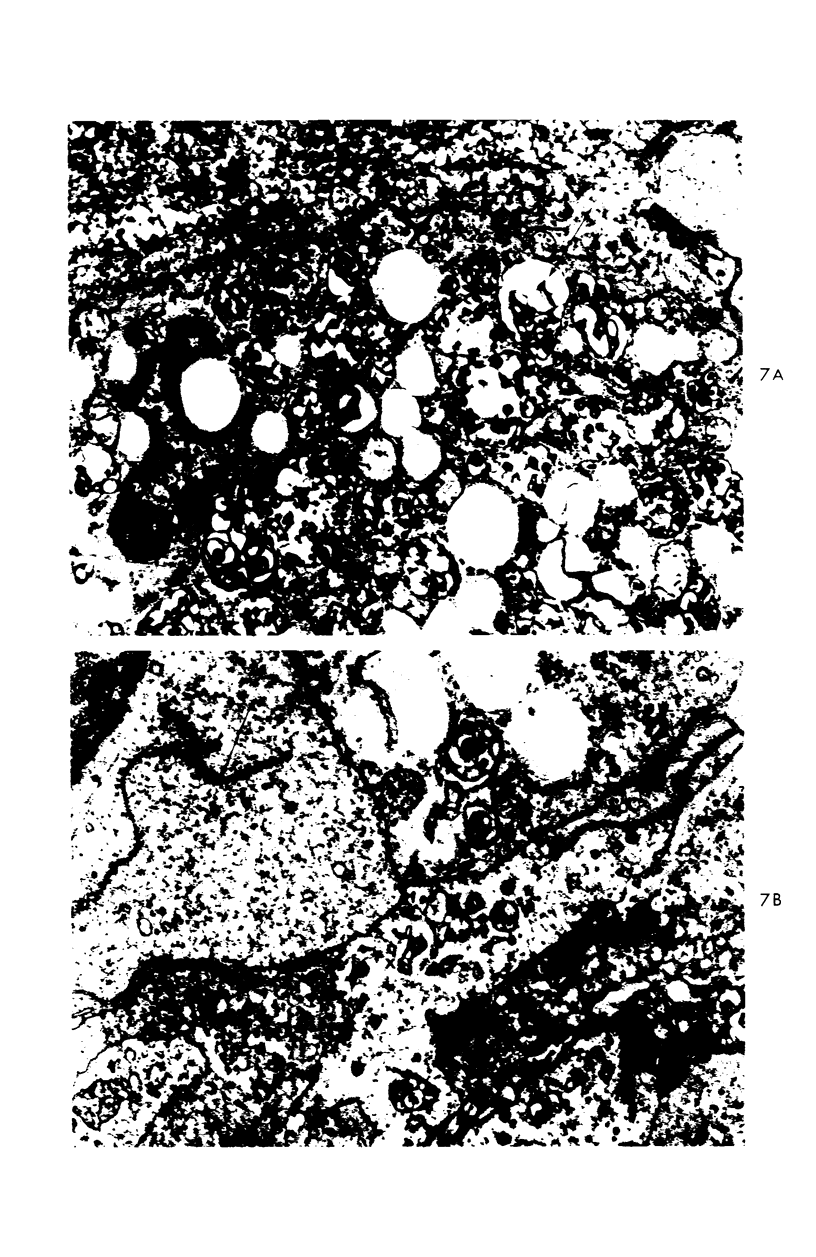
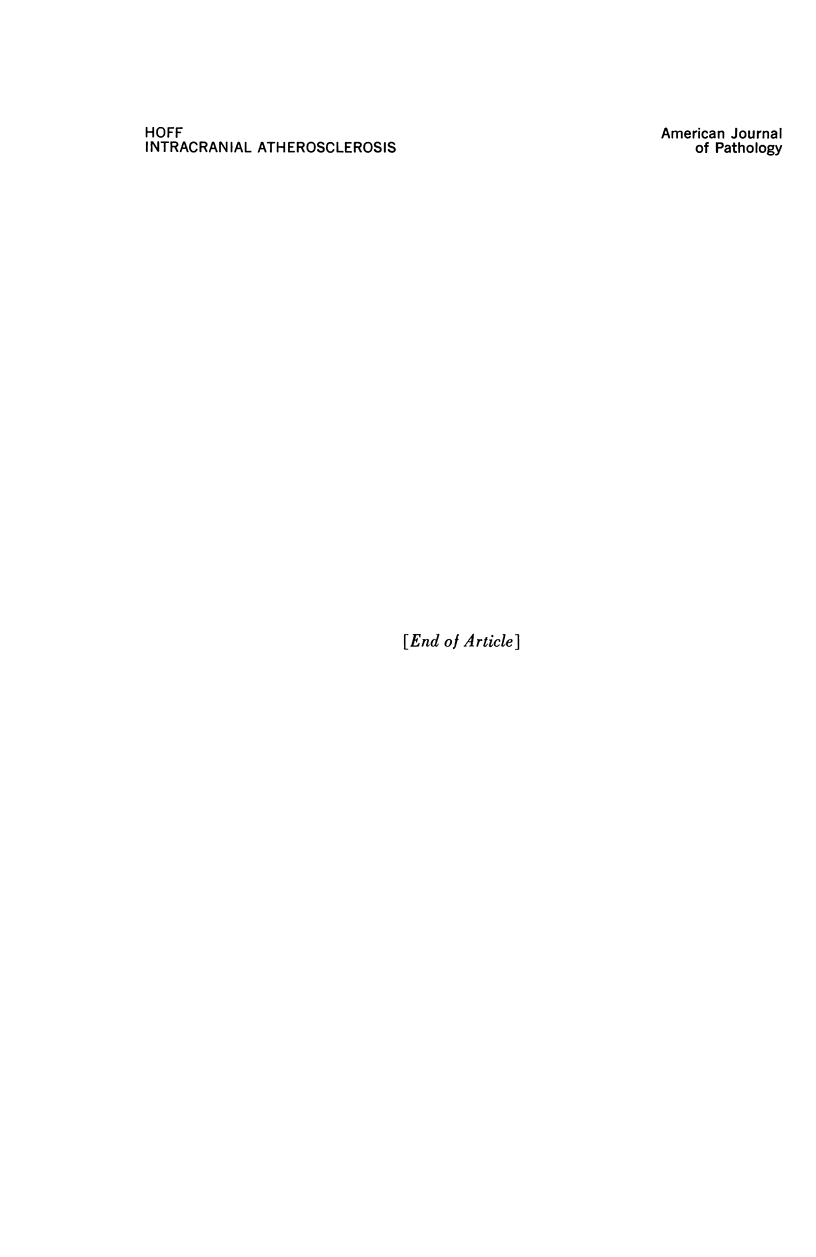
A descriptive study of atherosclerotic lesions in human intracranial arteries was undertaken using both light and electron microscopic technics. Arterial segments of human middle cerebral, internal carotid and basilar arteries with gross fatty streak lesions were obtained at autopsy within 4 hours after death, fixed and embedded in plastic. A new technic has been developed in which 0.5 μ-thick sections can be stained with PAS/alcian blue, thereby enabling clear differentiation, substantiated by simultaneous electron microscopic studies, between the lipid-filled smooth muscle cell, which is surrounded by a PAS-positive basement membrane, and the lipid-filled blood monocyte. These technics demonstrated that the gross fatty streak comprised a focal intimal thickening bordered on the lumen by a patent endothelial lining and on the media by a usually intact elastic membrane. The lesion contained smooth muscle cells and cells presumed to be blood monocytes, both filled with and devoid of lipid droplets. Although little fragmented or new elastica was found in the extracellular space, numerous foci of membranous material could be observed, believed to be cell debris and plasma lipoproteins. The similarity between the morphology of such lesions in intracranial arteries with those found in other arterial beds suggests that the documented differences in the prevalence of atherosclerosis in the various beds has no bearing on either the structure of each type of lesion or its progression.
Full text
PDF









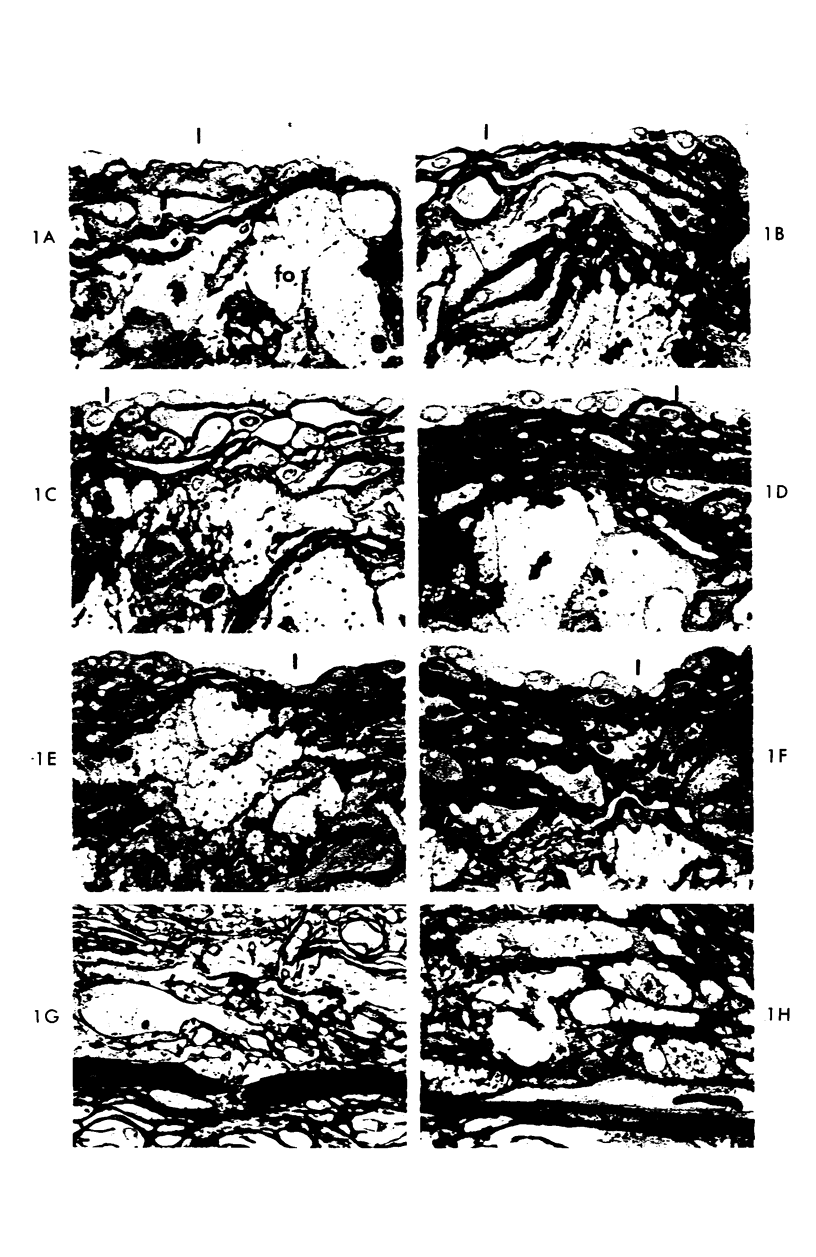
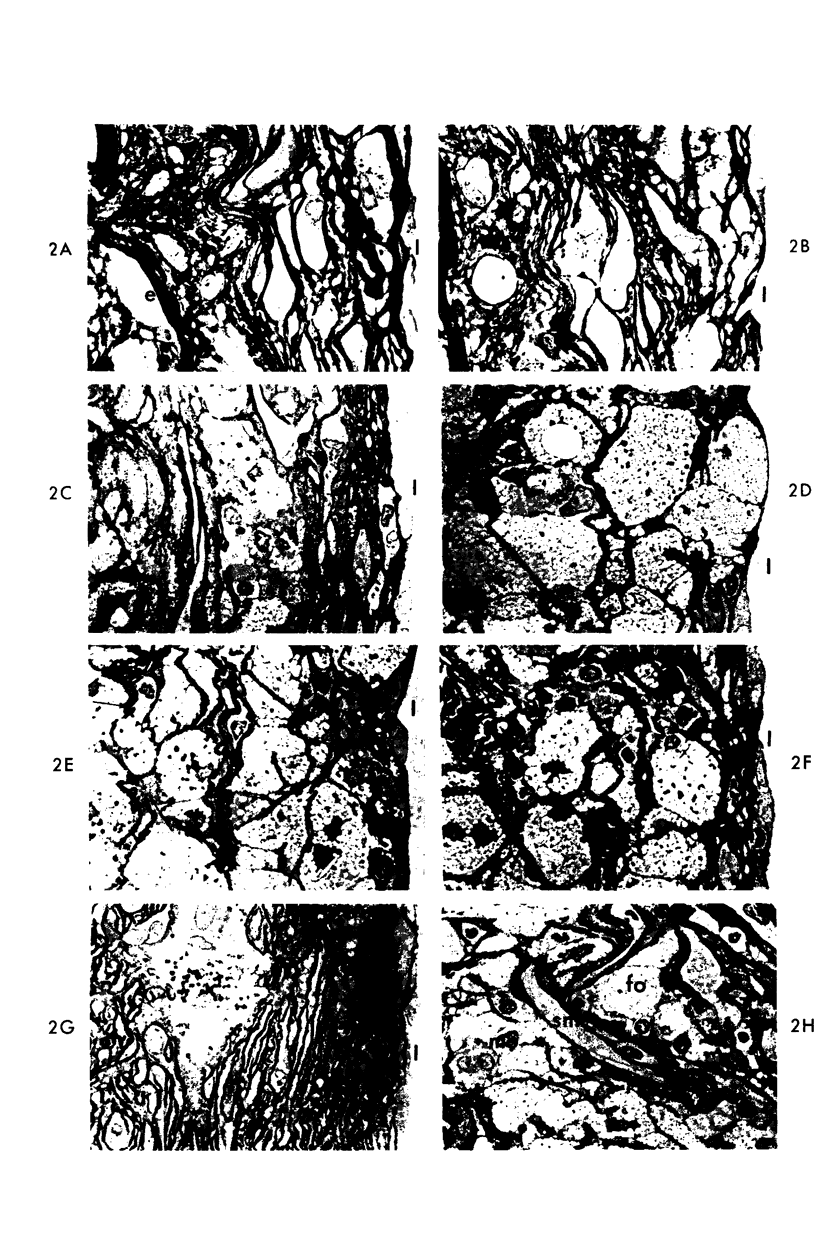
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER A. B., IANNONE A. Cerebrovascular disease. I. The large arteries of the circle of Willis. Neurology. 1959 May;9(5):321–332. doi: 10.1212/wnl.9.5.321. [DOI] [PubMed] [Google Scholar]
- DAY A. J. THE MACROPHAGE SYSTEM, LIPID METABOLISM AND ATHEROSCLEROSIS. J Atheroscler Res. 1964 Mar-Apr;4:117–130. doi: 10.1016/s0368-1319(64)80032-6. [DOI] [PubMed] [Google Scholar]
- Flora G., Dahl E., Nelson E. Electron microscopic observations on human intracranial arteries. Changes seen with aging and atherosclerosis. Arch Neurol. 1967 Aug;17(2):162–173. doi: 10.1001/archneur.1967.00470260052006. [DOI] [PubMed] [Google Scholar]
- Geer J. C., Catsulis C., McGill H. C., Jr, Stron J. P. Fine structure of the baboon aortic fatty streak. Am J Pathol. 1968 Feb;52(2):265–286. [PMC free article] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Ghidoni J. J., O'Neal R. M. Recent advances in molecular pathology: a review ultrastructure of human atheroma. Exp Mol Pathol. 1967 Dec;7(3):378–400. doi: 10.1016/0014-4800(67)90049-4. [DOI] [PubMed] [Google Scholar]
- Grunnet M. Changes in cerebral arteries with aging. Arch Pathol. 1969 Sep;88(3):314–318. [PubMed] [Google Scholar]
- Haust M. D., Geer J. C. Mechanism of calcification in spontaneous aortic arteriosclerotic lesions of the rabbit. An electron microscopic study. Am J Pathol. 1970 Sep;60(3):329–346. [PMC free article] [PubMed] [Google Scholar]
- Haust M. D. The morphogenesis and fate of potential and early atherosclerotic lesions in man. Hum Pathol. 1971 Mar;2(1):1–29. doi: 10.1016/s0046-8177(71)80019-9. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Gottlob R. Ultrastructural changes of large rabbit blood vessels following mild mechanical trauma. Virchows Arch A Pathol Pathol Anat. 1968;345(2):93–106. doi: 10.1007/BF00548644. [DOI] [PubMed] [Google Scholar]
- Hsieh H. H. Cerebrovascular disease: a comparative study of cerebral and visceral arteries. Neurology. 1967 Aug;17(8 Pt 1):752–762. doi: 10.1212/wnl.17.8.752. [DOI] [PubMed] [Google Scholar]
- Imai H., Thomas W. A. Cerebral atherosclerosis in swine: role of necrosis in progression of diet-induced lesions from proliferative to atheromatous stage. Exp Mol Pathol. 1968 Jun;8(3):330–357. doi: 10.1016/s0014-4800(68)80004-8. [DOI] [PubMed] [Google Scholar]
- Lie J. T., Brown A. L., Jr, Carter E. T. Spectrum of aging changes in temporal arteries. Its significance, in interpretation of biopsy of temporal artery. Arch Pathol. 1970 Sep;90(3):278–285. [PubMed] [Google Scholar]
- MAYOR H. D., HAMPTON J. C., ROSARIO B. A simple method for removing the resin from epoxy-embedded tissue. J Biophys Biochem Cytol. 1961 Apr;9:909–910. doi: 10.1083/jcb.9.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. 3. The cholesterol-induced lesions and the effect of a cholesterol and oil diet on the preexisting spontaneous plaque in the chicken aorta. Lab Invest. 1970 Nov;23(5):521–535. [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- Newman H. A., Murad T. M., Geer J. C. Foam cells of rabbit atheromatous lesion. Identification and cholesterol uptake in isolated cells. Lab Invest. 1971 Dec;25(6):586–595. [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker F., Odland G. F. A correlative histochemical, biochemical and electron microscopic study of experimental atherosclerosis in the rabbit aorta with special reference to the myo-intimal cell. Am J Pathol. 1966 Feb;48(2):197–239. [PMC free article] [PubMed] [Google Scholar]
- Peterson M., Day A. J., Tume R. K., Eisenberg E. Ultrastructure, fatty acid content, and metabolic activity of foam cells and other fractions separated from rabbit atherosclerotic lesions. Exp Mol Pathol. 1971 Oct;15(2):157–169. doi: 10.1016/0014-4800(71)90096-7. [DOI] [PubMed] [Google Scholar]
- Scott R. F., Jones R., Daoud A. S., Zumbo O., Coulston F., Thomas W. A. Experimental atherosclerosis in rhesus monkeys. II. Cellular elements of proliferative lesions and possible role of cytoplasmic degeneration in pathogenesis as studied by electron microscopy. Exp Mol Pathol. 1967 Aug;7(1):34–57. doi: 10.1016/0014-4800(67)90037-8. [DOI] [PubMed] [Google Scholar]
- Solberg L. A., Eggen D. A. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971 May;43(5):711–724. doi: 10.1161/01.cir.43.5.711. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E. Intimal proliferation and spontaneous lipid deposition in the cerebral arteies of sheep and steers. J Atheroscler Res. 1965 Nov-Dec;5(6):556–568. doi: 10.1016/s0368-1319(65)80032-1. [DOI] [PubMed] [Google Scholar]
- Still W. J., Dennison S. M. Reaction of the arterial intima of the rabbit to trauma and hyperlipemia. Exp Mol Pathol. 1967 Apr;6(2):245–253. doi: 10.1016/0014-4800(67)90060-3. [DOI] [PubMed] [Google Scholar]
- Suzuki M. Experimental cerebral atherosclerosis in the dog. I. A morphologic study. Am J Pathol. 1972 May;67(2):387–402. [PMC free article] [PubMed] [Google Scholar]
- Tucker C. F., Catsulis C., Strong J. P., Eggen D. A. Regression of early cholesterol-induced aortic lesions in rhesus monkeys. Am J Pathol. 1971 Dec;65(3):493–514. [PMC free article] [PubMed] [Google Scholar]
- Wurster N. B., Zilversmit D. B. The role of phagocytosis in the development of atherosclerotic lesions in the rabbit. Atherosclerosis. 1971 Nov-Dec;14(3):309–322. doi: 10.1016/0021-9150(71)90060-8. [DOI] [PubMed] [Google Scholar]
- ZUGIBE F. T., BROWN K. D. Histochemical studies in atherogenesis. Human cerebral arteries. Circ Res. 1961 Jul;9:897–905. doi: 10.1161/01.res.9.4.897. [DOI] [PubMed] [Google Scholar]