Abstract
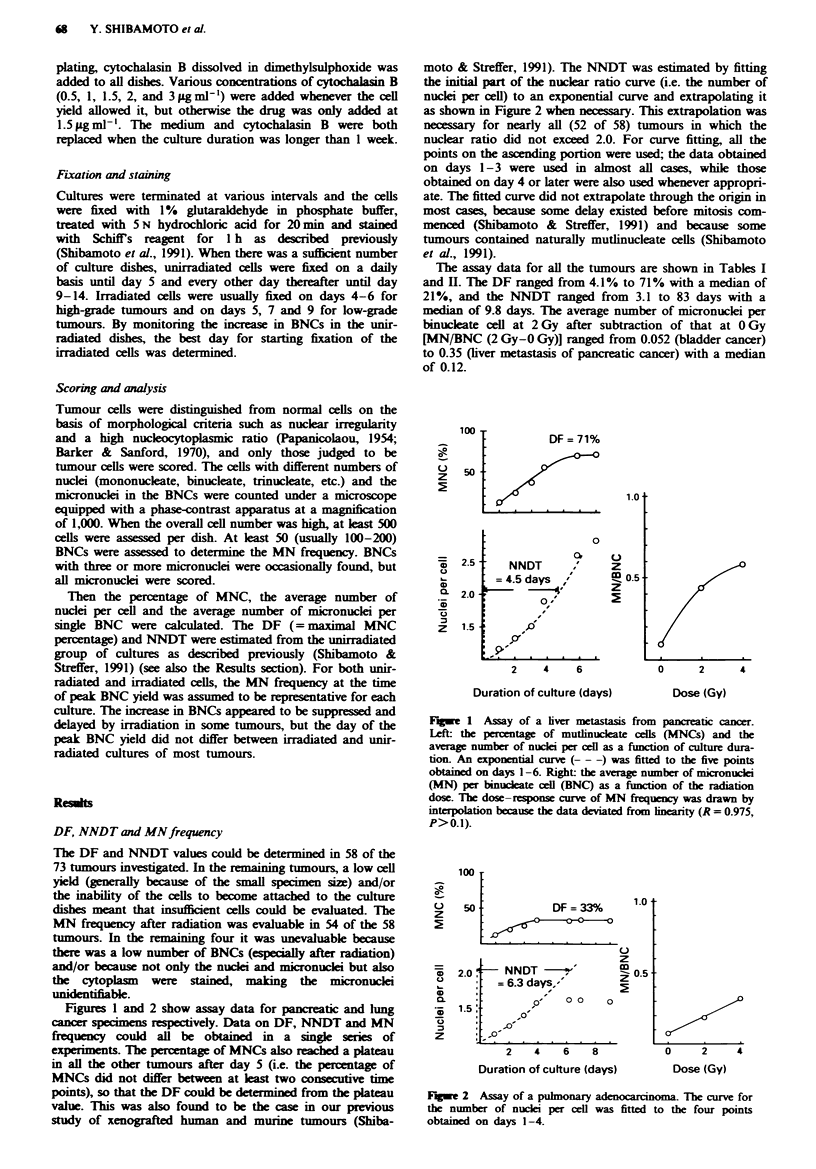
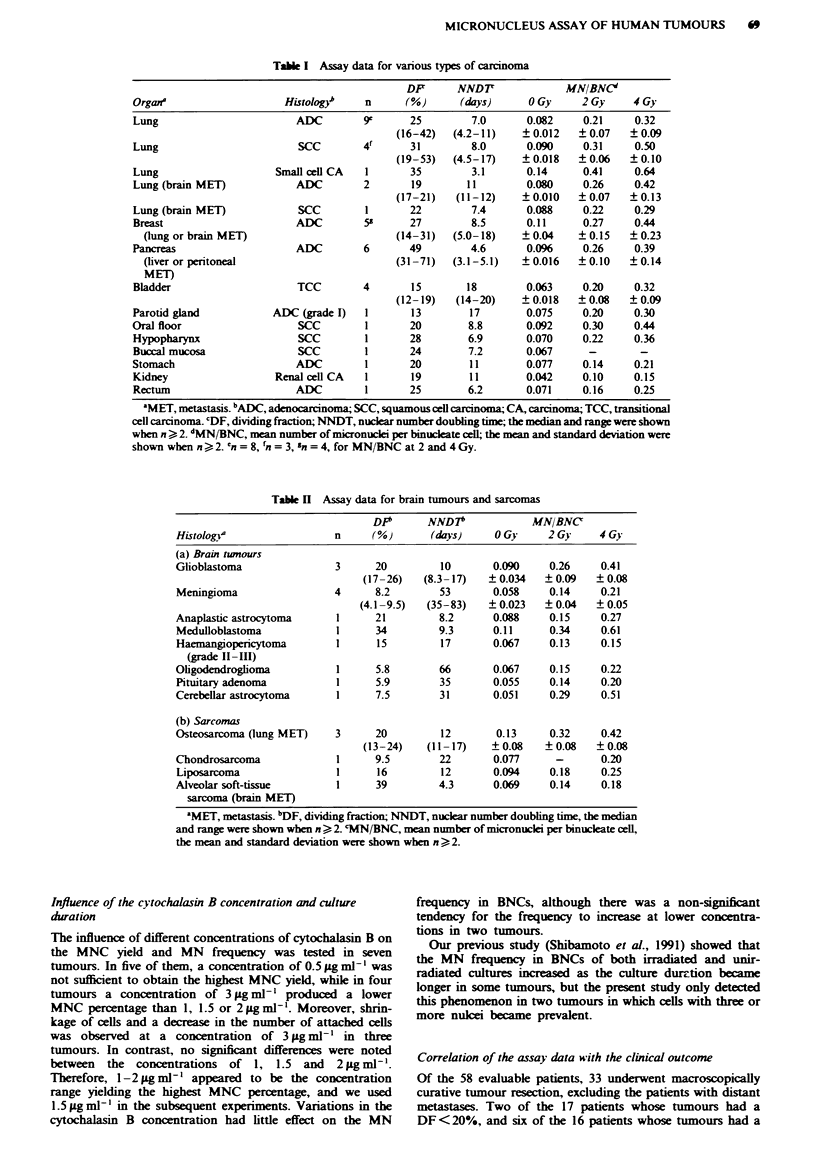
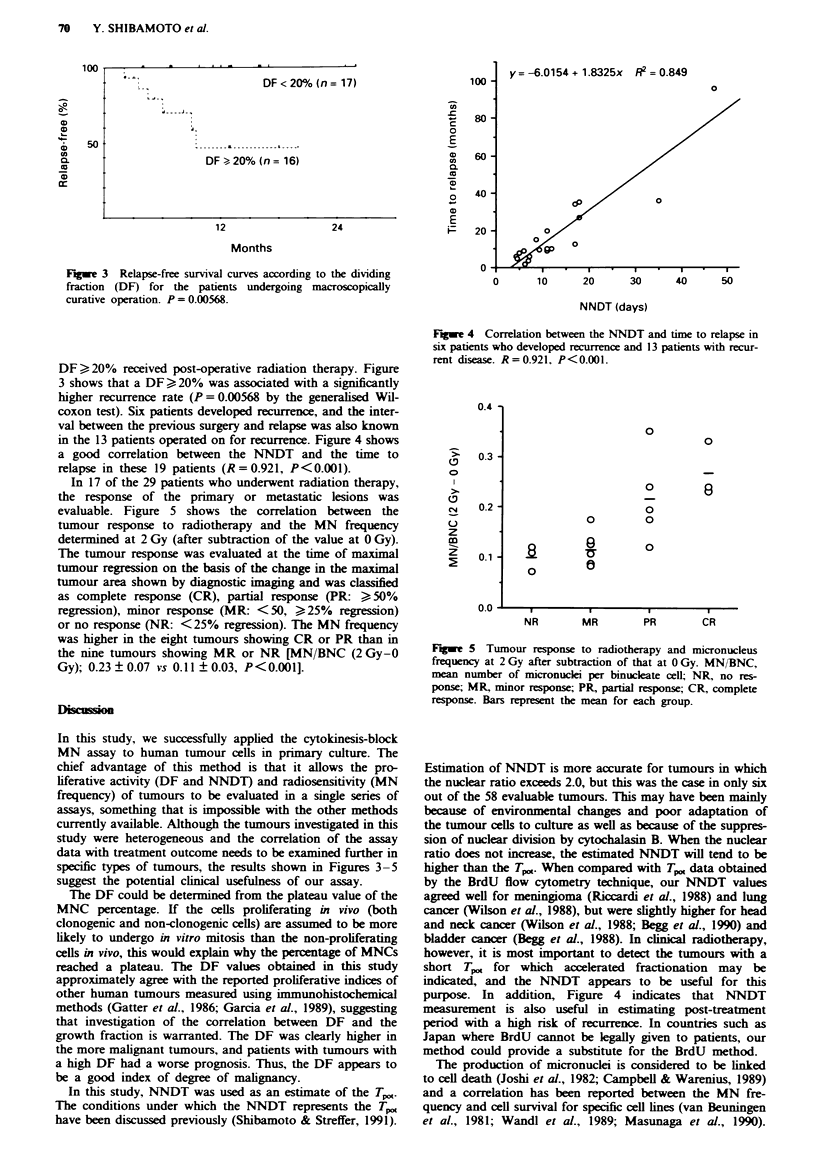
We established an in vitro cytokinesis-block micronucleus assay of human tumours for estimation of the proportion of cells undergoing mitosis (the dividing fraction, DF), the time for the number of nuclei to double and the radiosensitivity in terms of the micronucleus frequency, based on a concept described previously. Under certain conditions, the nuclear number doubling time (NNDT) was considered to represent the potential doubling time. Tumour specimens obtained at surgery were disaggregated into single-cell suspensions and were directly cultured in the presence of cytochalasin B with or without irradiation. At various intervals, the percentage of multinucleate cells (the plateau value represented the DF), the average number of nuclei per cell and the number of micronuclei in binucleate cells were determined. DF and NNDT values were obtained in 58 of the 73 tumours investigated, and the micronucleus frequency was obtained in 54 of these 58 tumours. The DF ranged from 4.1% to 71% and the NNDT ranged from 3.1 to 83 days. A DF > or = 20% was associated with a higher recurrence rate in patients undergoing curative operation. A correlation was found between the NNDT and the time to relapse in patients with recurrent disease. The average number of micronuclei per binucleate cell at 2 Gy of irradiation (after subtraction of the value at 0 Gy) ranged from 0.052 to 0.35. Tumours which produced more micronuclei after irradiation showed a better response to radiotherapy. This assay can be readily performed on human tumours and appears to have promise as a predictive assay for radiation therapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker B. E., Sanford K. K. Cytologic manifestations of neoplastic transformation in vitro. J Natl Cancer Inst. 1970 Jan;44(1):39–63. [PubMed] [Google Scholar]
- Begg A. C., Hofland I., Moonen L., Bartelink H., Schraub S., Bontemps P., Le Fur R., Van Den Bogaert W., Caspers R., Van Glabbeke M. The predictive value of cell kinetic measurements in a European trial of accelerated fractionation in advanced head and neck tumors: an interim report. Int J Radiat Oncol Biol Phys. 1990 Dec;19(6):1449–1453. doi: 10.1016/0360-3016(90)90357-p. [DOI] [PubMed] [Google Scholar]
- Brock W. A., Baker F. L., Peters L. J. Radiosensitivity of human head and neck squamous cell carcinomas in primary culture and its potential as a predictive assay of tumor radiocurability. Int J Radiat Biol. 1989 Nov;56(5):751–760. doi: 10.1080/09553008914552001. [DOI] [PubMed] [Google Scholar]
- Campbell I. R., Warenius H. M. Radiation-induced cell death by chromatin loss. A model to explain the shape of low-linear-energy-transfer cell survival curves. Br J Radiol. 1989 Apr;62(736):338–343. doi: 10.1259/0007-1285-62-736-338. [DOI] [PubMed] [Google Scholar]
- Fenech M., Morley A. A. Measurement of micronuclei in lymphocytes. Mutat Res. 1985 Feb-Apr;147(1-2):29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Dunnill M. S., Gerdes J., Stein H., Mason D. Y. New approach to assessing lung tumours in man. J Clin Pathol. 1986 Jun;39(6):590–593. doi: 10.1136/jcp.39.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Joshi G. P., Nelson W. J., Revell S. H., Shaw C. A. X-ray-induced chromosome damage in live mammalian cells, and improved measurements of its effects on their colony-forming ability. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Feb;41(2):161–181. doi: 10.1080/09553008214550171. [DOI] [PubMed] [Google Scholar]
- Masunaga S., Ono K., Wandl E. O., Fushiki M., Abe M. Use of the micronucleus assay for the selective detection of radiosensitivity in BUdR-unincorporated cells after pulse-labelling of exponentially growing tumour cells. Int J Radiat Biol. 1990 Aug;58(2):303–311. doi: 10.1080/09553009014551641. [DOI] [PubMed] [Google Scholar]
- Peters L. J., Brock W. A., Johnson T., Meyn R. E., Tofilon P. J., Milas L. Potential methods for predicting tumor radiocurability. Int J Radiat Oncol Biol Phys. 1986 Apr;12(4):459–467. doi: 10.1016/0360-3016(86)90053-2. [DOI] [PubMed] [Google Scholar]
- Riccardi A., Danova M., Wilson G., Ucci G., Dörmer P., Mazzini G., Brugnatelli S., Girino M., McNally N. J., Ascari E. Cell kinetics in human malignancies studied with in vivo administration of bromodeoxyuridine and flow cytometry. Cancer Res. 1988 Nov 1;48(21):6238–6245. [PubMed] [Google Scholar]
- Shibamoto Y., Streffer C. Estimation of the dividing fraction and potential doubling time of tumors using cytochalasin B. Cancer Res. 1991 Oct 1;51(19):5134–5138. [PubMed] [Google Scholar]
- Shibamoto Y., Streffer C., Fuhrmann C., Budach V. Tumor radiosensitivity prediction by the cytokinesis-block micronucleus assay. Radiat Res. 1991 Dec;128(3):293–300. [PubMed] [Google Scholar]
- Shibamoto Y., Streffer C., Sasai K., Oya N., Abe M. Radiosensitization efficacy of KU-2285, RP-170 and etanidazole at low radiation doses: assessment by in vitro cytokinesis-block micronucleus assay. Int J Radiat Biol. 1992 Apr;61(4):473–478. doi: 10.1080/09553009214551231. [DOI] [PubMed] [Google Scholar]
- Wandl E. O., Ono K., Kain R., Herbsthofer T., Hienert G., Höbarth K. Linear correlation between surviving fraction and the micronucleus frequency. Int J Radiat Biol. 1989 Nov;56(5):771–775. doi: 10.1080/09553008914552031. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dische S., Saunders M. I., Des Rochers C., Lewis A. A., Bennett M. H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1988 Oct;58(4):423–431. doi: 10.1038/bjc.1988.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beuningen D., Streffer C., Bertholdt G. Mikronukleusbildung im Vergleich zur Uberlebensrate von menschlichen Melanomzellen nach Röntgen-, Neutronenbestrahlung und Hyperthermie. Strahlentherapie. 1981 Sep;157(9):600–606. [PubMed] [Google Scholar]


