Abstract
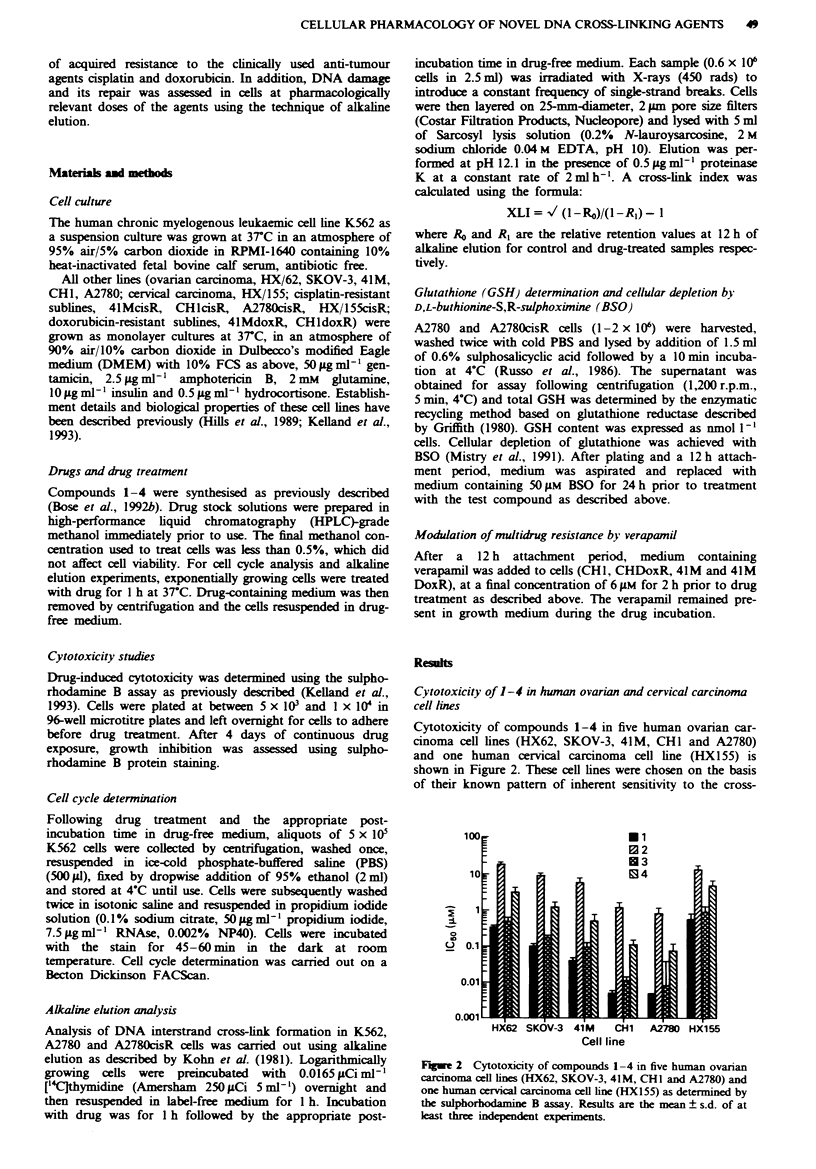
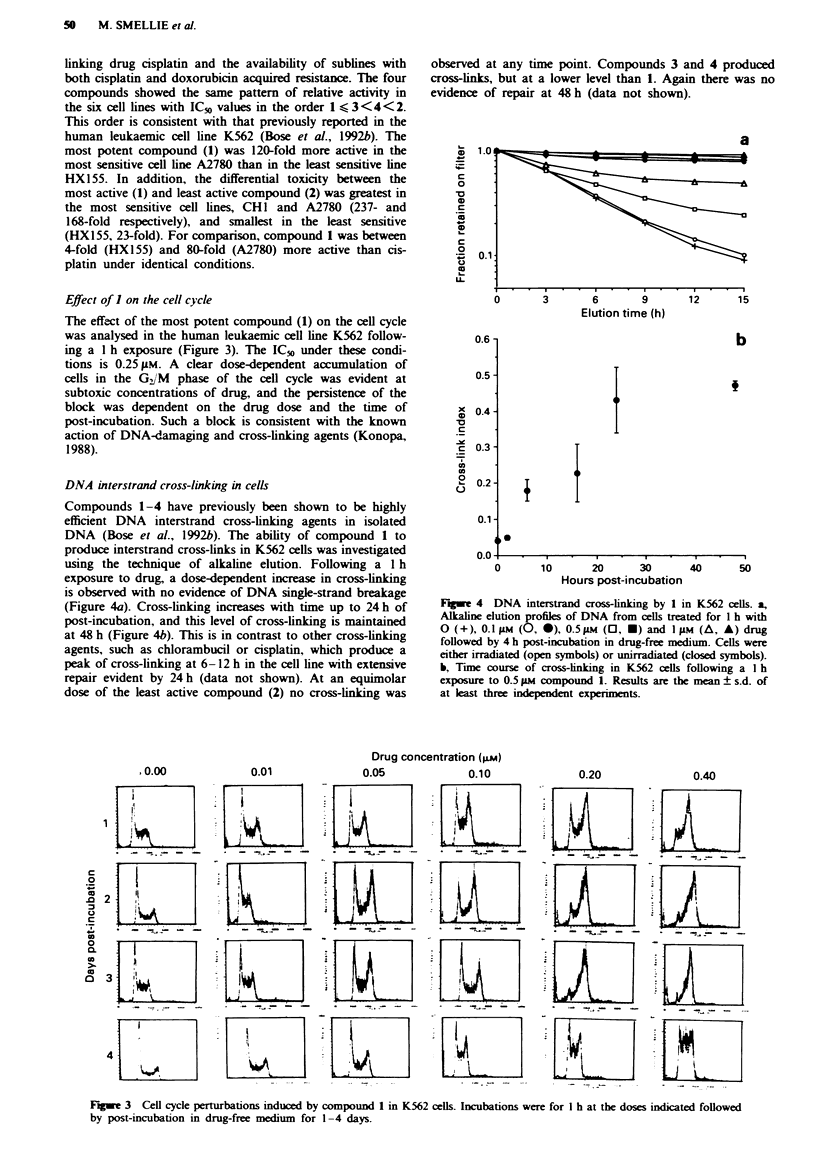
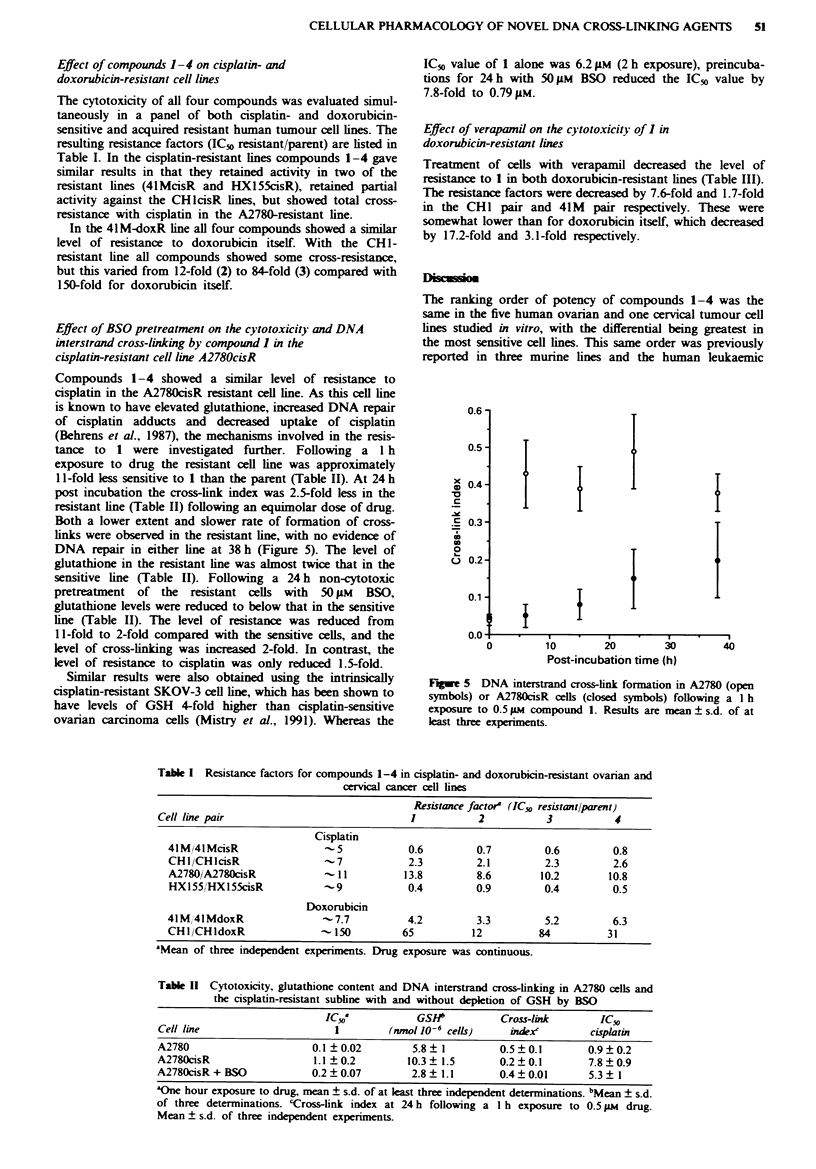
The cellular pharmacology of a series of C8-linked pyrrolobenzodiazepine dimers with polymethylene linkers of n = 3-6 (compounds 1-4) has been studied in a range of human tumour cell lines. The four compounds showed the same pattern of relative activity in five ovarian carcinoma cell lines and one cervical carcinoma cell line with the order of IC50 values of 1 < or = 3 < 4 < 2, which correlated with the previously demonstrated DNA interstrand cross-linking ability of the compounds in plasmid DNA. In human leukaemic K562 cells the agents produced a block in the G2/M phase of the cell cycle characteristic of cross-linking drugs, and extensive interstrand cross-linking was observed in cells by alkaline elution with no evidence of single-strand breaks. Cross-links continued to increase up to 24 h following a 1 h exposure to drug, and no repair was evident by 48 h. In a series of ovarian and cervical carcinoma cell lines with acquired resistance to cisplatin no cross-resistance to the most potent compound 1 was observed in two lines whose major mechanism of resistance to cisplatin was reduced platinum transport. Cross-resistance to 1 was observed in a cell line (A2780cisR) possessing elevated glutathione, and depletion of intracellular glutathione using D,L-buthionine-S,R-sulphoximine (BSO) from 10.25 nmol to 2.8 nmol 10(-6) cells reduced the level of resistance from 11-fold to 2-fold compared with sensitive cells. Cross-linking in the resistant cells was restored to 80% of the level in the parent line by BSO pretreatment. There was also a correlation between glutathione levels and sensitivity to 1 measured in several other ovarian cell lines. Compound 1 also showed cross-resistance in the doxorubicin-resistant cell line 41MdoxR and partial cross-resistance in CH1doxR cells. Both these lines possess elevated levels of p170 glycoprotein. Following treatment with 6 microM verapamil, the resistance in these lines decreased almost 2-fold and 8-fold respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedford P., Fox B. W. DNA-DNA interstrand crosslinking by dimethyanesulphonic acid esters. Correlation with cytotoxicity and antitumour activity in the Yoshida lymphosarcoma model and relationship to chain length. Biochem Pharmacol. 1983 Aug 1;32(15):2297–2301. doi: 10.1016/0006-2952(83)90176-4. [DOI] [PubMed] [Google Scholar]
- Behrens B. C., Hamilton T. C., Masuda H., Grotzinger K. R., Whang-Peng J., Louie K. G., Knutsen T., McKoy W. M., Young R. C., Ozols R. F. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987 Jan 15;47(2):414–418. [PubMed] [Google Scholar]
- Brox L. W., Gowans B., Belch A. L-phenylalanine mustard (melphalan) uptake and cross-linking in the RPMI 6410 human lymphoblastoid cell line. Cancer Res. 1980 Apr;40(4):1169–1172. [PubMed] [Google Scholar]
- Burt R. K., Poirier M. C., Link C. J., Jr, Bohr V. A. Antineoplastic drug resistance and DNA repair. Ann Oncol. 1991 May;2(5):325–334. doi: 10.1093/oxfordjournals.annonc.a057949. [DOI] [PubMed] [Google Scholar]
- Erickson L. C., Bradley M. O., Ducore J. M., Ewig R. A., Kohn K. W. DNA crosslinking and cytotoxicity in normal and transformed human cells treated with antitumor nitrosoureas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):467–471. doi: 10.1073/pnas.77.1.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. P., Hecht S. M., Reynolds V. L., Molineux I. J., Hurley L. H. DNA sequence specificity of the pyrrolo[1,4]benzodiazepine antitumor antibiotics. Methidiumpropyl-EDTA-iron(II) footprinting analysis of DNA binding sites for anthramycin and related drugs. Biochemistry. 1986 Mar 25;25(6):1249–1258. doi: 10.1021/bi00354a009. [DOI] [PubMed] [Google Scholar]
- Hills C. A., Kelland L. R., Abel G., Siracky J., Wilson A. P., Harrap K. R. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989 Apr;59(4):527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. H., Reck T., Thurston D. E., Langley D. R., Holden K. G., Hertzberg R. P., Hoover J. R., Gallagher G., Jr, Faucette L. F., Mong S. M. Pyrrolo[1,4]benzodiazepine antitumor antibiotics: relationship of DNA alkylation and sequence specificity to the biological activity of natural and synthetic compounds. Chem Res Toxicol. 1988 Sep-Oct;1(5):258–268. doi: 10.1021/tx00005a002. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Abel G., McKeage M. J., Jones M., Goddard P. M., Valenti M., Murrer B. A., Harrap K. R. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 1993 Jun 1;53(11):2581–2586. [PubMed] [Google Scholar]
- Kelland L. R., Mistry P., Abel G., Loh S. Y., O'Neill C. F., Murrer B. A., Harrap K. R. Mechanism-related circumvention of acquired cis-diamminedichloroplatinum(II) resistance using two pairs of human ovarian carcinoma cell lines by ammine/amine platinum(IV) dicarboxylates. Cancer Res. 1992 Jul 15;52(14):3857–3864. [PubMed] [Google Scholar]
- Konopa J. G2 block induced by DNA crosslinking agents and its possible consequences. Biochem Pharmacol. 1988 Jun 15;37(12):2303–2309. doi: 10.1016/0006-2952(88)90355-3. [DOI] [PubMed] [Google Scholar]
- Mellish K. J., Kelland L. R., Harrap K. R. In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer. 1993 Aug;68(2):240–250. doi: 10.1038/bjc.1993.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. J., Thurston D. E., Nevell T. G. Evaluation of the electrophilicity of DNA-binding pyrrolo[2,1-c][1,4]benzodiazepines by HPLC. J Antibiot (Tokyo) 1990 Oct;43(10):1286–1292. doi: 10.7164/antibiotics.43.1286. [DOI] [PubMed] [Google Scholar]
- O'Connor P. M., Kohn K. W. Comparative pharmacokinetics of DNA lesion formation and removal following treatment of L1210 cells with nitrogen mustards. Cancer Commun. 1990;2(12):387–394. doi: 10.3727/095535490820873949. [DOI] [PubMed] [Google Scholar]
- Pera M. F., Jr, Rawlings C. J., Shackleton J., Roberts J. J. Quantitative aspects of the formation and loss of DNA interstrand crosslinks in Chinese hamster cells following treatment with cis-diamminedichloroplatinum(II) (cisplatin). II. Comparison of results from alkaline elution, DNA renaturation and DNA sedimentation studies. Biochim Biophys Acta. 1981 Sep 28;655(2):152–166. doi: 10.1016/0005-2787(81)90005-8. [DOI] [PubMed] [Google Scholar]
- Russo A., DeGraff W., Friedman N., Mitchell J. B. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986 Jun;46(6):2845–2848. [PubMed] [Google Scholar]
- Sorenson C. M., Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988 Aug 15;48(16):4484–4488. [PubMed] [Google Scholar]
- Sunters A., Springer C. J., Bagshawe K. D., Souhami R. L., Hartley J. A. The cytotoxicity, DNA crosslinking ability and DNA sequence selectivity of the aniline mustards melphalan, chlorambucil and 4-[bis(2-chloroethyl)amino] benzoic acid. Biochem Pharmacol. 1992 Jul 7;44(1):59–64. doi: 10.1016/0006-2952(92)90038-k. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]
- Zwelling L. A., Filipski J., Kohn K. W. Effect of thiourea on survival and DNA cross-link formation in cells treated with platinum(II) complexes, L-phenylalanine mustard, and bis(2-chloroethyl)methylamine. Cancer Res. 1979 Dec;39(12):4989–4995. [PubMed] [Google Scholar]
- Zwelling L. A., Kohn K. W., Ross W. E., Ewig R. A., Anderson T. Kinetics of formation and disappearance of a DNA cross-linking effect in mouse leukemia L1210 cells treated with cis- and trans-diamminedichloroplatinum(II). Cancer Res. 1978 Jun;38(6):1762–1768. [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Schwartz H., Dobson P. P., Kohn K. W. DNA cross-linking as an indicator of sensitivity and resistance of mouse L1210 leukemia to cis-diamminedichloroplatinum(II) and L-phenylalanine mustard. Cancer Res. 1981 Feb;41(2):640–649. [PubMed] [Google Scholar]


