Abstract
A regiochemical and stereochemical mixture of flexible linkers bearing terminal azide functionality was synthesized in two steps from squalene and was used to connect two high affinity NDP-α-MSH ligands or two low affinity MSH(4) ligands. The ligands were N-terminally acylated using N-hydroxysuccinimidoyl 5-hexynoate and were subsequently attached to the linker via copper-catalyzed “click” 3+2 cyclization of the azide and alkyne moieties. In vitro biological evaluations showed that the binding affinity to the human melanocortin 4 receptor was not diminished for most linker-ligand combinations relative to the corresponding parental ligand. Statistical and cooperative binding effects were observed for dimeric constructs containing the low affinity ligand MSH(4), but not for dimeric NDP-α-MSH constructs, presumably due to slow off rates for this high affinity ligand.
Early detection of many human cancers would be facilitated by the availability of reagents that could seek out and selectively bind to cancer cells and report their existence and location by non-invasive molecular imaging.1 Our strategy for development of such reagents involves linking reporter moieties to multivalent ligands that contain multiple copies of individual binding units that hence cooperatively bind to cell surface receptors that are overexpressed in cancer cells.2 Multivalent molecules should display enhanced affinity and selectivity for such cells.2a,3
The foundation for ligand-guided multivalent attachment of reporter moieties to cell surfaces bearing targeted receptors was laid in part by studies that employed a poly(vinyl alcohol) (PVA) scaffold decorated with fluorescein and NDP-α-MSH ligands.4 Such molecules bound specifically and irreversibly to mouse and human melanoma cells that expressed and displayed melanocortin receptors. The PVA-based system was not extended to other peptide hormone/receptor systems due to problems with the attachment chemistry and the insolubility of PVA. Recent advances in polymer-supported multivalent binding3b,5 have prompted a reexamination of this earlier approach with the intent of developing a more soluble biocompatible polymer scaffold and more efficient ligand attachment chemistry. Herein we present model synthetic and in vitro biological studies relevant to these goals.
The copper-catalyzed 3+2 cyclization of azide and alkyne moieties to generate triazole products6 was an obvious choice to replace the maleimide electrophile/thiol nucleophile and thiol/disulfide redox attachment chemistries used previously with PVA.4
The search for a PVA replacement was guided by the observation that incompletely hydrolyzed PVA is often more water-soluble at room temperature than is a more completely hydrolyzed PVA.7 This is presumably due to interruption of hydrogen bonded microcrystalline domains, and suggested that a polymer bearing fewer, stereorandom hydroxyl groups might exhibit less crystallinity and greater water solubility. Such a polymer can be prepared from polyisoprene.8 Since analysis of a polymer product is complicated by high molecular weight and polydispersity, we chose to employ squalene as a more tractable model system for the establishment of the synthetic methodology necessary for this approach.
Hydroboration and oxidation of squalene9 produced a mixture of hexaols 1 (Scheme 1).10 The crude product was purified by column chromatography (67% yield) and analyzed by NMR and HRMS. These analyses confirmed that secondary alcohols were highly predominant and that a mixture of diastereomers had been produced.11 Reaction of 1 with 2.2 equivalents of sodium hydride in DMF, followed by addition of 4.0 equivalents of l-azido-8-bromohexane (2)12 afforded, after chromatography, monoazides 3 and bisazides 4 in 38% and 24% yields, respectively, as mixtures of regioisomers. Trace amounts of trisazides and higher homologs were also obtained.
Scheme 1.
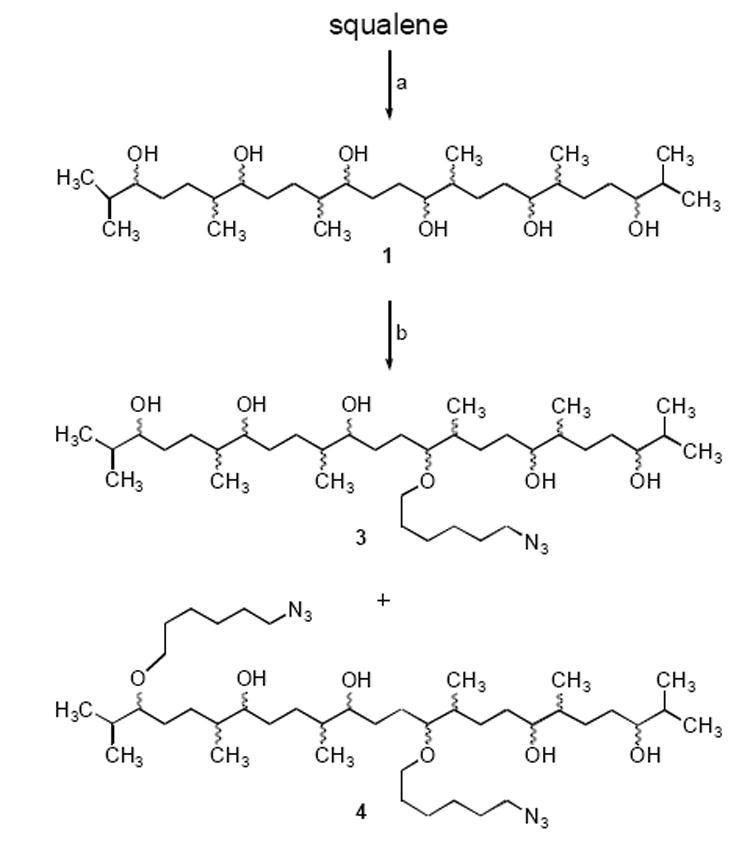
Reagents: (a) BH3, THF; H2O2, NaOH. (b) NaH, DMF; Br(CH2)6N3 (2). The sites of attachment of the 6-azidohexyl moieties to the squalane scaffold shown are arbitrary. Mixtures of all possible stereoisomers of 1 and egioisomers of 3 and 4 are produced.
To demonstrate the feasibility of click attachment to 3 and 4, alkyne 7 was prepared from serine amide hydrochloride (5)13 and 2,5-dioxopyrrolidin-1-yl hex-5-ynoate (6, Scheme 2). Reaction of 3 and 4 with 7 in the presence of the catalyst derived from tetrakis(acetonitrile)copper(1) hexafluorophosphate and tris-(benzyltriazolylmethyl)-amine (TBTA, 8)14 in t-BuOH/water 2:1 gave the corresponding triazoles 9a and 10a (Figure 1) in 84% and 80% yields, respectively, after chromatography.
Scheme 2.
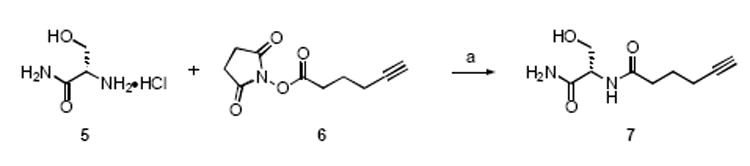
Reagents: (a) Et3N, DMF, H2O.
Figure 1.
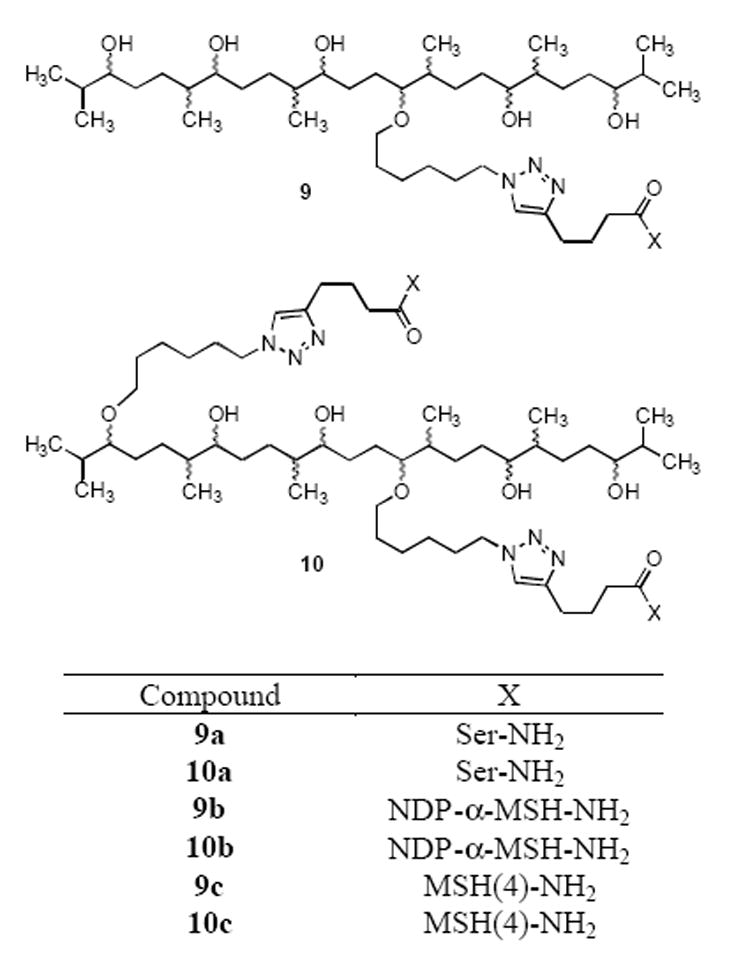
Monovalent and divalent compounds on a squalene-derived scaffold prepared via “click” attachment of alkynylated ligands. The sites of ligand attachment shown are arbitrary.
The high affinity ligand NDP-α-MSH15 was constructed on Rink amide Tentagel S resin (initial loading 0.17 mmol/g).16 The product resin retained all side chain protecting groups. NHS ester 6 was coupled to the N-terminus of the resin-bound peptide. Simultaneous side chain deprotection and cleavage of the peptide from the resin was effected using a mixture of trifluoroacetic acid, 1,2-ethanedithiol, thioanisole, and water (91/3/3/3), producing the desired alkynylated NDP-α-MSH ligand 11. An alkynylated derivative, 12, of the low affinity ligand MSH(4)17 was similarly prepared. Compounds 11 and 12 were purified by reverse-phase C18 preparative HPLC and were characterized by ESI-MS and MALDI-TOF. Details appear in Table 1.
Table 1.
Mass spectral and HPLC characterization of compounds 9b-c, 10b-c, 11, and 12
| Compd | Formula | Calc Mass
[Ion] |
Mass Found
(error) |
tRa | K’ |
|---|---|---|---|---|---|
| 9b | C118H188N24O25 | 781.4804
[M+3]3+ |
781.4833
(3.7 ppm) |
35.3-36.4 | nab |
| 10b | C206H314N48O44 | 1042.1030
[M+4]4+ |
1042.1055
(2.4 ppm) |
33.4-34.7 | nab |
| 9c | C74H120N14O11 | 691.4709
[M+2]2+ |
691.4693
(2.4 ppm) |
34.8-37.3 | nab |
| 10c | C118H178N28O16 | 748.8070
[M+3]3+ |
748.8052
(2.4 ppm) |
32.9-36.9 | nab |
| 11 | C82H115N21O19 | 566.9638
[M+3]3+ |
566.9653
(2.6 ppm) |
20.41 | 8.66 |
| 12 | C38H47N11O5 | 738.3840
[M+1]1+ |
738.3829
(1.4 ppm) |
15.48 | 6.02 |
Linear gradient of from 10-60% CH3CN in 0.1% aqueous TFA over 50 min.
Not applicable, this compound is a regiochemical and stereochemical mixture.
Reaction of azides 3 and 4 with 11 in methanol in the presence of the copper/TBTA catalyst gave the corresponding triazoles 9b and 10b, while reaction of 3 and 4 with 12 gave the corresponding triazoles 9c and 10c (Figure 1). These compounds were purified by preparative reverse-phase C18 HPLC and were characterized by analytical HPLC and by ESI-MS and MALDI-TOF. Details appear in Table 1.
HEK293 cells overexpressing the human melanocortin 4 receptor (hMC4R) were used to assess ligand binding,18 which was evaluated using a previously described lanthanide (Eu) based competitive binding assay.19,20 Table 2 lists the IC50 values (averaged over n experiments) for the serine amide-containing compounds 7, 9a, and 10a, the ligand NDP-α-MSH and the NDP-α-MSH-containing compounds 11, 9b, and 10b, and the ligand MSH(4) and the MSH(4)-containing compounds 12, 9c and 10c.
Table 2.
Competitive binding of NDP-α-MSH, MSH(4), 7, 9a-c, 10a-c, 11, and 12 to hMC4R
| Compounds | IC50 | na |
|---|---|---|
| 7 | nab | 3 |
| 9a | nab | 3 |
| 10a | nab | 3 |
| NDP-α-MSH | 5.9 ± 1.9 nM | 5 |
| 11 | 3.2 ± 0.9 nM | 4 |
| 9b | 3.9 ± 1.2 nM | 5 |
| 10b | 3.3 ± 0.8 nM | 6 |
| MSH(4) | 1.1 ± 0.5 μM | 4 |
| 12 | 0.9 ± 0.1 μM | 4 |
| 9c | 3.3 ± 0.5 μM | 7 |
| 10c | 0.4 ± 0.2 μM | 7 |
The IC50 value given is the average of n independent binding experiments, each done in quadruplicate.
This compound was unable to displace Eu-NDP-α-MSH in the concentration range tested (10-5-10-12 M).
As expected, serine amide derivatives 7, 9a, and 10a were ineffective at displacing Eu-NDP-α-MSH over the range of concentrations tested (10-5-10-12 M).
The IC50 values for compounds 9b [scaffold + NDP-α-MSH], 10b [NDP-α-MSH + scaffold + NDP-α-MSH], and the alkynylated NDP-α-MSH control compound 11 were all somewhat lower than the value for the parental NDP-α-MSH ligand. These results indicate that acylation of the N-terminus of NDP-α-MSH modestly enhanced binding to hMC4R, and that attachment of NDP-α-MSH to the squalene-derived scaffold had no effect on the binding. No statistical halving of the IC50 was observed for 10b relative to 9b. This is presumably due to slow release of the ligand from the receptor, as previously described for short, rigid linkers.21
In contrast, the IC50 values for compounds 9c [scaffold + MSH(4)] and 10c [MSH(4) + scaffold + MSH(4)] were higher and lower, respectively, than the values for the alkynylated MSH(4) control compound 12 and the parental MSH(4) ligand, which were similar. These results indicate that acylation of the N-terminus of MSH(4) had little or no effect on the binding to hMC4R, that attachment of MSH(4) to the squalene-derived scaffold had a modest detrimental effect on the binding, and that statistical and proximity effects resulted in the lowering of the IC50 for 10c relative to 9c by an order of magnitude.
This model study has demonstrated that a poly(1,5-diol) can be prepared from a polyisoprene (squalene) and modified by alkylation with a 1-azido-ω-bromoalkane to introduce terminal azide moieties; that functionally-protected peptides on a solid support can be N-terminally acylated with active ω-alkynyl esters to introduce alkyne moieties that survive deprotection and cleavage from the resin; that the above azides and alkynes can be joined by copper-catalyzed 3+2 cyclization to form triazoles that can be purified and characterized; and that attachment in this manner of NDP-α-MSH and MSH(4) ligands to the polyisoprene-derived scaffold does not significantly interfere with ligand binding to the hMC4 receptor at the cell surface. Extension of this work to polymeric polyisoprene-derived scaffolds and to other ligand/receptor combinations is underway.
Supplementary Material
Acknowledgments
TBTA was a generous gift from Professor K. B. Sharpless of The Scripps Research Institute. This work was supported by grants R33 CA 95944, RO1 CA 97360, and P30 CA 23074 from the National Cancer Institute.
Footnotes
This paper is dedicated to Professor C. Dale Poulter on the occasion of his 65th birthday.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Gillies RJ, Hoffman JM, Lam KS, Menkens AE, Piwnica-Worms DR, Sullivan DC, Weissleder R. Molecular Imaging. 2005;4:98–103. doi: 10.1162/15353500200505115. [DOI] [PubMed] [Google Scholar]; Gillies RJ, Hruby VJ. Expert Opin Ther Targets. 2003;7:137–139. doi: 10.1517/14728222.7.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Handl HL, Vagner J, Han H, Mash E, Hruby VJ, Gillies RJ. Expert Opin Ther Targets. 2004;8:565–586. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- 3.Mammen M, Chio S-K, Whitesides GM. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; Kiessling LL, Gestwicki JE, Strong LE. Angew Chem Int Ed. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma SD, Granberry ME, Jiang J, Leong SLP, Hadley ME, Hruby VJ. Bioconjugate Chem. 1994;5:591–601. doi: 10.1021/bc00030a015. [DOI] [PubMed] [Google Scholar]; Sharma SD, Jiang J, Hadley ME, Bentley DL, Hruby VJ. Proc Natl Acad Sci USA. 1996;93:13715–13720. doi: 10.1073/pnas.93.24.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairo CW, Gestwicki JE, Kanai M, Kiessling LL. J Am Chem Soc. 2002;124:1615–1619. doi: 10.1021/ja016727k. [DOI] [PubMed] [Google Scholar]; Griffith BR, Allen BL, Rapraeger AC, Kiessling LL. J Am Chem Soc. 2004;126:1608–1609. doi: 10.1021/ja037646m. [DOI] [PubMed] [Google Scholar]; Li RC, Broyer RM, Maynard HD. J Polym Sci Part A: Polym Chem. 2006;44:5004–5013. [Google Scholar]
- 6.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Moore WRAD, O’Dowd M. In: Properties and Applications of Polyvinyl Alcohol. Finch CA, editor. Staples Printers Ltd; Kent, England: 1968. pp. 77–87. [Google Scholar]
- 8.Levesque G, Pinazzi C. Bull Soc Chim Fr. 1971:1008–1010. [Google Scholar]; Yamaguchi H, Azuma K, Minoura Y. Polym J. 1972;3:12–20. [Google Scholar]
- 9.Purchased from Aldrich Chemical Company
- 10.Isacesco N, Taleb-Bendiab S-A, Chatzopoulos M, Montheard JP, Vergnaud JM. C R Acad Sci Ser C: Chim. 1974;279:683–686. [Google Scholar]
- 11.In some runs, hydroboration was apparently incomplete. Hydrogen peroxide converted the remaining alkene moieties into epoxides, which were ring-opened by hydroxide, resulting in contamination of the hexaol 1 by heptaol and octaol impurities (from HPLC and HRMS)
- 12.Shon Y-S, Kelly KF, Halas NJ, Lee TR. Langmuir. 1999;15:5329–5332. [Google Scholar]
- 13.Purchased from Senn Chemicals USA
- 14.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 15.The “high-affinity” ligand employed here was based on NDP-α-MSH (Ser-Tyr-Ser-Nle-Glu-His-dPhe-Arg-Trp-Gly-Lys-Pro-Val);; see Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. Proc Natl Acad Sci USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754.Hadley ME, Anderson B, Heward CB, Sawyer TK, Hruby VJ. Science. 1981;213:1025–1027. doi: 10.1126/science.6973820.
- 16.Merrifield RB. J Am Chem Soc. 1963;85:2149–2154. [Google Scholar]; Hruby VJ, Meyer J-P. In: Bioorganic Chemistry: Peptides and Proteins. Hecht SM, editor. Oxford Univ. Press; New York: 1998. pp. 27–64. [Google Scholar]
- 17.The “low-affinity” ligand employed was based on the minimal active sequence for full agonist activity of α-MSH (His-dPhe-Arg-Trp);; see Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, de Lauro Castrucci AM, Hintz MF, Riehm JP, Rao KR. J Med Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033.Castrucci AML, Hadley ME, Sawyer TK, Wilkes BC, Al-Obiedi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao KR, Hruby VJ. Gen Comp Endocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x.Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C, Gantz I. J Med Chem. 1997;40:2133–2139. doi: 10.1021/jm960840h.
- 18.The hMC4R vector was originally received from Dr. Ira Gantz;; see Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. J Biol Chem. 1993;268:15174–15179.
- 19.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Anal Biochem. 2004;330:242–250. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Binding assay: HEK293/hMC4R cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS. Cells were plated in Black & White Isoplates (Wallac, 1450-583) at a density of 12,000 cells/well and were allowed to grow for 3 days. On the day of the experiment, media was aspirated from all wells. Ligands of interest were diluted in binding buffer (DMEM, 1mM 1,10-phenanthroline, 200 mg/L Bacitracin 0.5 mg/L Leupeptin, 0.3% BSA) to result in final dilutions ranging from 10 μM to 4 pM. Eu-labeled NDP-α-MSH was used at a final concentration of 10 nM. 50 μL of the ligand of interest and 50 μL of Eu-NDP-α-MSH were added to each well and plates were incubated for 40 min at 37 °C. Following the incubation, cells were washed 4 times with Wash Buffer (50 mM Tris-HCl, 0.2% BSA, 30 mM NaCl), enhancement solution (Perkin Elmer, 1244-105) was added (100 μL/well), and the plates were incubated for 30 min at 37 °C prior to reading. The plates were read on a Wallac VICTOR3 instrument using the standard Eu TRF measurement (340 nm excitation, 400 μsec delay, and emission collection for 400 μsec at 615 nm). Competition curves were analyzed with GraphPad Prism Software using the sigmoidal dose-response classical equation for nonlinear regression analysis. Each data point represents the average of 4 samples, with the error bars indicating standard error of the mean.
- 21.Vagner J, Handl HL, Monguchi Y, Jana U, Begay LJ, Mash EA, Hruby VJ, Gillies RJ. Bioconjugate Chem. 2006;17:1545–1550. doi: 10.1021/bc060154p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


