Abstract
The ability to extract meaningful information from transcriptome technologies such as cDNA microarrays relies on the precision, sensitivity and reproducibility of the measured values for a given gene across multiple samples. Given the lack of a ‘gold standard’ for the production of microarrays using current technologies, there is a high degree of variation in the quality of data derived from microarray experiments. Poor reproducibility not only adds to the cost of a given study but also leads to data sets that are difficult to interpret. For glass slide DNA microarrays, much of this variation is introduced systematically, during the spotting, or deposition, of the DNA onto the slide surface. In order to reduce this type of systematic variation we tested spotting solutions containing different detergent additives in the presence of one of two different denaturants and determined their effect on spot quality. We show that spotting cDNA in a solution consisting of the zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulfonate (CHAPS) in the presence of formamide or dimethyl sulfoxide yields spots of superior quality in terms of morphology, size homogeneity and signal reproducibility, as well as overall intensity, when used with popular, commercially available slides.
INTRODUCTION
During the last decade, several high throughput, genome-wide technologies have emerged that allow the determination of relative mRNA expression levels for a large number of genes (1–3). This type of approach has yielded important advances in both the basic sciences and the pharmaceutical industry in areas ranging from metabolic and signaling pathways to tumor classification and drug discovery. Using a glass microarray format this is achieved by hybridizing fluorescently labeled targets from different samples to arrays of spots containing oligonucleotides or cDNAs corresponding to the genes to be studied (4–6). The validity of the biological conclusions derived from microarray experiments hinges upon the accuracy and precision of the data. Non-genetic influences such as the quality of the spotted DNA, differences in the labeling reactions and/or batch effects associated with a particular sample can add variation or noise to an experiment and greatly bias the final analysis of the data (7–10). The morphology of a given spot, which can affect reproducibility, can be optimized by using agents that affect the homogeneity, ionic strength and/or surface tension of the DNA solution. In addition, different glass surface chemistries have been developed in order to optimize the attachment of the DNA to the surface of the slide and to maximize the amount of DNA available for hybridization. For all types of interactions, the most common spotting solutions reported in the literature are either salt based, such as saline sodium citrate (SSC), phosphate-based buffers in the presence or absence of additives [50% dimethyl sulfoxide (DMSO) or betaine] or DMSO alone (11–13). However, these spotting solutions often lead to heterogeneous spot morphologies (e.g. ‘doughnuts’ and ‘bulls eyes’), deposition inconsistencies and/or oversized spots. Many of these problems are compounded not only by several ‘operational’ factors during the spotting procedure, such as sample loss due to evaporation, but also by independent factors that often cannot be controlled by the end user such as the homogeneity of the slide surface. In order to optimize the spotting procedure we tested the effects of different detergent additives in the presence of different denaturants (DMSO or formamide) on spot quality, as assessed by spot morphology, and reproducibility, as well as on overall signal intensity above background, and compared the results to those obtained with other previously published spotting solutions. Here we present the results of the effects of 20 different solution compositions on the spotting of a variety of DNAs corresponding to open reading frames (ORFs) derived from Saccharomyces cerevisiae and Escherichia coli. We found that the zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulfonate (CHAPS) in the presence of formamide greatly improves the reproducibility, morphology and the calculated signal intensity of the spotted DNA after hybridization of fluorescently labeled samples.
MATERIALS AND METHODS
Generation of microarrays
Twenty-four ORFs from S.cerevisiae were chosen based both on theoretical predictions of codon usage (http://genome-www.stanford.edu/Saccharomyces/; 14–16) as well as on experimentally derived data showing that these ORFs exhibited a wide range of expression levels in cells growing under standard culture conditions (17). All ORFs were amplified by PCR and purified using QiaQuick PCR clean-up columns (Qiagen SA, Courteboeuf, France). The PCR products ranged in size from 300 to 700 bp. The purified DNAs were quantified and arrayed into 384-well source plates at final concentrations of 100 ng/µl in final volumes of 8 µl of water. The DNAs were dried using centrifugation under vacuum and resuspended in 8 µl of one of 20 different spotting solutions. The compositions of the 20 spotting solutions as well as the detailed information concerning the 24 ORFs that were chosen for the study are summarized in Tables 1 and 2, respectively. All detergent additives and denaturants were obtained from Sigma (St Louis, MO). As an initial screen, four ORFs were arrayed in triplicate with each of the 20 solutions. Based on the results from this screen we chose the six best spotting solutions to study the complete set of genes (24 ORFs) under different conditions (different pin sizes and 4 months after storage at 4°C). For these tests all 24 ORFs were arrayed in quadruplicate on each slide and data from two slides per condition were used for the analysis.
Table 1. Description of the spotting solution tested.
| Number | Additivea | Concentration | Denaturanta |
|---|---|---|---|
| 1 | Triton X-100 (CMC = 0.2–0.9 mM)b | 0.08 mM (0.005%) | D |
| 2 | 0.08 mM (0.005%) | F | |
| 3 | 1.6 mM (0.1%) | D | |
| 4 | 1.6 mM (0.1%) | F | |
| 5 | DOC (CMC = 2–6 mM) | 1.2 mM (0.05%) | D |
| 6 | 1.2 mM (0.05%) | F | |
| 7 | 24 mM (1%) | D | |
| 8 | 24 mM (1%) | F | |
| 9 | CHAPS (CMC = 6–10 mM) | 1.6 mM (0.1%) | D |
| 10 | 1.6 mM (0.1%) | F | |
| 11 | 16 mM (1%) | D | |
| 12 | 16 mM (1%) | F | |
| 13 | CTAB (CMC = 1 mM) | 0.27 mM (0.01%) | D |
| 14 | 0.27 mM (0.01%) | F | |
| 15 | 2.7 mM (0.1%) | D | |
| 16 | 2.7 mM (0.1%) | F | |
| 17 | None | N/A | D |
| 18 | None | N/A | F |
| 19 | Betaine/SSC | 1.5 M/3× | N/A |
| 20 | SSC | 3× | N/A |
aFour different detergent additives, above and below their CMC, were tested in the presence of either DMSO (D) or formamide (F).
bCMC values are based on calculations assuming a 50 mM Na+ concentration. Solutions 17 and 18 are DMSO and formamide alone, respectively. Betaine (1.5 M) in the presence of 3× SSC as previously described (15) was tested, as well as 3× SSC alone.
Table 2. Information concerning the 24 S.cerevisiae ORFs.
| ORF name | Gene name | Codon bias | CAI codon usage index | Copies/cell | PCR product size (bp) | Patch |
|---|---|---|---|---|---|---|
| YKL058w | TOA2 | –0.08 | 0.10 | 2.70 | 261 | 1 |
| YKL044w | n/a | 0.03 | 0.10 | 0.03 | 290 | 2 |
| YKL066w | n/a | –0.14 | 0.07 | 0.90 | 392 | 3 |
| YKL213c | DOA1 | 0.04 | 0.13 | 1.10 | 600 | 4 |
| YKL185w | ASH1 | 0.03 | 0.12 | 1.20 | 598 | 5 |
| YKL003c | MRP17 | 0.05 | 0.12 | 1.60 | 328 | 6 |
| YKL092c | BUD2 | 0.04 | 0.14 | 0.20 | 516 | 7 |
| YKL074c | MUD2 | 0.10 | 0.13 | 1.00 | 615 | 8 |
| YKL012w | PRP40 | 0.04 | 0.13 | 1.20 | 590 | 9 |
| YKL165c | MCD4 | 0.10 | 0.16 | 1.90 | 634 | 10 |
| YKL189w | HYM1 | 0.10 | 0.15 | 0.60 | 679 | 11 |
| YKL114c | APN1 | 0.06 | 0.14 | 1.20 | 700 | 12 |
| YKL211c | TRP3 | 0.22 | 0.18 | 2.50 | 534 | 13 |
| YKL007w | CAP1 | 0.16 | 0.18 | 1.50 | 584 | 14 |
| YKL184w | SPE1 | 0.17 | 0.18 | 1.20 | 537 | 15 |
| YKL016c | ATP7 | 0.25 | 0.22 | 2.80 | 450 | 16 |
| YKL192c | ACP1 | 0.32 | 0.21 | 6.20 | 328 | 17 |
| YKL142w | MRP8 | 0.24 | 0.20 | 1.50 | 547 | 18 |
| YKL001c | MET14 | 0.34 | 0.27 | 1.30 | 473 | 19 |
| YKL138c | MRPL31 | 0.24 | 0.23 | 1.00 | 362 | 20 |
| YKL216w | URA1 | 0.24 | 0.23 | 16.20 | 535 | 21 |
| YKL060c | FBA1 | 0.94 | 0.87 | 74.10 | 685 | 22 |
| YKL056c | n/a | 0.83 | 0.73 | 47.40 | 439 | 23 |
| YKL182w | FAS1 | 0.48 | 0.36 | 8.40 | 687 | 24 |
ORF names are indicated with accompanying gene names where available (n/a refers to an ORF where no gene name has been attributed). Codon bias, CAI codon adaptation index and copies of a given ORF per yeast cell are described in Materials and Methods.
For the experiments using E.coli DNA, PCR products corresponding to the 4288 predicted ORFs in the E.coli genome DNA were obtained from Sigma-Genosys (20–50 µl containing from 16 to 150 ng/µl), dried using vacuum centrifugation and resuspended with CHAPS (1%) and formamide (50%) at a maximal final DNA concentration of 100 ng/µl.
DNAs were arrayed onto activated CMT-GAPs slides (Corning Inc., Corning, NY) using the GeneTAC G3 robot (Genomics Solutions, Huntington, UK) in a controlled environment (20°C and 30% humidity). Unless otherwise specified, 200 µm pins were used for all experiments. After spotting, the slides were incubated overnight, protected from the light, at room temperature. The DNA was then UV-crosslinked to the slide using 250 mJ/cm2 (Hoefer UVC500; Amersham Pharmacia Biotech, San Francisco, CA). For quality control purposes we incubated an extra slide from every experiment with SYBR Green II (Molecular Probes, Eugene, OR) diluted 1:10 000. These slides were preprocessed under identical conditions as slides destined for hybridization (see below).
Yeast whole-genome slides (CMT™ Yeast-S228c Gene Arrays) were purchased from Corning (Corning Life Sciences, Corning, NY). Only spots whose intensities were greater than background were included in the analyses.
RNA extraction and fluorescent labeling of cDNA and genomic DNA
Total RNA from S.cerevisiae homozygous diploid strain CN011 (no auxotrophic markers) cultured under conditions of fermentation (2% glucose medium, F) or respiration (2% glycerol medium, R) was prepared by the ‘hot phenol’ method as previously described (18). For the Series 2 experiments, which were completed 4 months after the initial study, new cultures were grown and RNA from each was extracted according to the above protocol. RNA (up to 80 µg) was labeled in batch [Cyanine 3 dye (Cy3) for the R sample or Cyanine 5 dye (Cy5) for the F sample] and divided into aliquots after purification and applied to replicate slides for hybridization. All labeling reactions were performed using the Micromax direct labeling system (Perkin Elmer Life Sciences, Albany, NY). The samples were purified by precipitation with isopropanol and 1 µl was analyzed by fractionation through a 1.2% agarose gel. The remainder of the samples were dried using centrifugation under vacuum and resuspended in the appropriate volume (110 µl/slide) of the hybridization buffer accompanying the Micromax direct labeling kit. The samples were denatured at 95°C for 3 min and applied directly to the array. For experiments involving E.coli DNA genome-wide arrays, total E.coli genomic DNA, prepared as previously described (19), was fragmented by digestion with HaeIII and fluorescently labeled with Cy3 or Cy5 dUTP dyes using Klenow and random hexamers as described by Bernstein and Cohen (20) and applied to the arrays as described above.
Array hybridization and image acquisition
All arrays were hybridized and washed identically using an automated hybridization station (Genomics Solutions). Before hybridization all slides were preprocessed identically by washing for 5 min with each of 2× SSC/0.1% SDS, 0.2× SSC and water and dried as described above. Fluorescently labeled samples were hybridized to the arrays using the following conditions: 65°C for 3 h; 55°C for 3 h; 50°C for 12 h. The equivalent of 20 µg of total RNA was used per slide per fluorophore. The slides were scanned using a GenePix 4000B Microarray Scanner equipped with 635 and 532 nm excitation lasers for Cy5 and Cy3, respectively, and the resulting 16 bit TIFF images were analyzed with the accompanying software (Axon Instruments Inc., Foster City, CA). All slides in a given experimental series were scanned using the same PMT voltage (650 V for Cy5 and 425 V for Cy3) and laser power (100%). The background-subtracted median pixel intensities (local median background pixel intensities defined and calculated by GenePix) was used as a measure of overall spot intensity. The coefficient of variation (CV) of pixel intensity (or spot size) was calculated by dividing the standard deviation of the pixel intensity (or spot diameter) by the mean pixel intensity (or spot diameter calculated from spot replicates) based on all of the pixels within a given spot. These measures were used to assess spot morphology homogeneity and size reproducibility, respectively. A similar calculation was used to measure signal reproducibility, but in this case the median pixel value was used to calculate the standard deviation and mean of spot replicates.
RESULTS AND DISCUSSION
In order to reduce the systematic biases and noise introduced by the spotting procedure we tested the effects of a variety of detergent additives used in spotting solutions on spot morphology, overall signal intensity and reproducibility. Based on the nature of the hydrophilic head group, the detergents can be classified into four different groups: cationic, anionic, zwitterionic and non-ionic. In our tests, we included a representative from each group as well as solutions that have been previously described: 50% DMSO alone, 3× SSC alone and 3× SSC in 1.5 M betaine (11,13). The specific detergents from each group were chosen based on their physical/chemical characteristics, such as the homogeneity of the detergent and a well-defined critical micelle concentration (CMC), as well as being inexpensive and readily available. In an initial screen we eliminated the detergents SDS, poly(oxyethylene)n sorbitane monolaurate (Tween-20) and C12E8, as they all led to a poor quality of spot morphology (data not shown). Here we present the results of the following detergents as representatives of the different groups: non-ionic, octylphenolpoly(ethyleneglycolether)n (Triton X-100); anionic, deoxycholate (DOC); zwitterionic, CHAPS; cationic, cetyltrimethyl ammonium bromide (CTAB). In addition, we also tested the effects of adding either 50% DMSO or 50% formamide to each of the detergents to both act as a denaturant and to reduce sample evaporation. Twenty spotting solutions were evaluated in all and are listed in Table 1. Each detergent was tested above and below its CMC. We also tested four different types of slide surface modification (aminosilane, poly-l-lysine, aldehyde and epoxy) from a variety of different manufacturers. In general, the results obtained were consistent with each other barring some minor differences at the level of overall background. All the data presented here were obtained using aminosilane CMT-GAPS slides, as they are one of the most commonly used commercial slides.
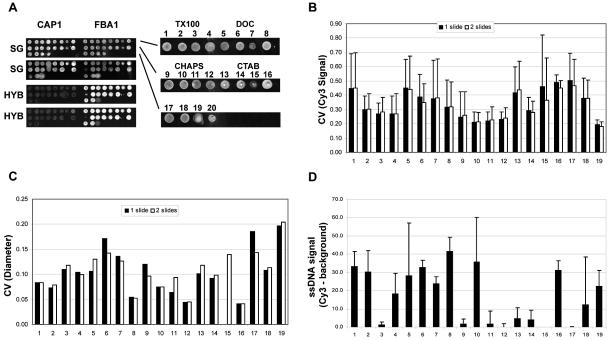
Of the 24 S.cerevisiae ORFs (see Table 2) an initial group of four ORFs (representing two low and two highly expressed genes) were selected to screen all 20 spotting solutions. Figure 1A shows the results obtained with two of the four ORFs, one low expressing gene (CAP1) and one high expressing gene (FBA1). We observed a high degree of variation in the morphology of the spots. The CV of the signal intensity (the Cy3 signal was used here and for all calculations hereafter as there was no detectable difference between the distribution of pixel intensities between Cy3 and Cy5) was calculated for each spot for all four ORFs. Figure 1B and C shows the average CV values for signal intensity and spot diameter for each spotting solution measured for all of the ORFs on one slide (n = 12) and for two different slides (n = 24), respectively. Barring CTAB, we observed an overall higher homogeneity of signal intensity of spots arrayed with detergents above their CMC. We did not observe such a relationship with CMC in respect of the reproducibility of spot diameter. The spotting solution consisting of 3× SSC alone (solution 20) was eliminated from the study due to problems of evaporation, as determined by the loss of material and missing spots. In agreement with previously published reports, DMSO, betaine and formamide all reduced the amount of evaporation compared to samples without these additives (data not shown). However, betaine (solution 19) yielded spot sizes that were, on average, very large and highly inconsistent. Formamide alone (solution 18), on the other hand, yielded more reproducibly sized spots with a higher signal homogeneity compared to betaine and DMSO alone. This observation was consistent with the data obtained for formamide compared to DMSO in the presence of the four different types of detergents. On the whole, DNA spotted in the zwitterionic detergent CHAPS (solutions 9–12) was found to have more homogeneous signals (lower average CV values for the signal intensity), as did the higher concentration of the non-ionic detergent Triton X-100 (solutions 3 and 4). Within these two groups of detergents, solutions 3, 4, 11 and 12 maintained higher levels of size reproducibility (a lower CV for spot diameter). The detergents by themselves had no effect on sample evaporation (data not shown). These solutions also did not contribute appreciably to non-specific signal, as the background-subtracted median pixel intensity measured for each control spot was less than one standard deviation (14 intensity units) away from the average local median background intensity (84 intensity units) measured for all of the spots over two slides (Fig. 1D).
Figure 1.
Spot quality assessment of 20 different spotting solutions. (A) Images of the two ORFS (CAP1 and FBA1) spotted using 20 different solutions on two different slides and either stained with SYBR Green II (SG) or after hybridization with fluorescently labeled cDNA (HYB). On the right is an example, at higher magnification, of the FBA1 spots stained with SG. (B) The average CVs (and the standard deviations) of the signal intensities obtained for Cy3 based on all of the spots for 19 of the 20 solutions across the two slides (n = 24). (C) The CVs of the spot diameters calculated for the spots for each solution on two replicate slides as shown in (B). (D) The average and standard deviation of the background-subtracted Cy3 signals (gray scale intensity units) obtained from negative control spots for each solution shown in (B) and (C) (n = 600).
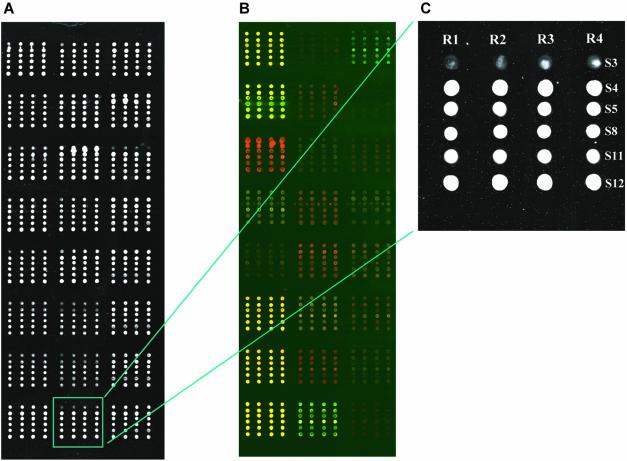
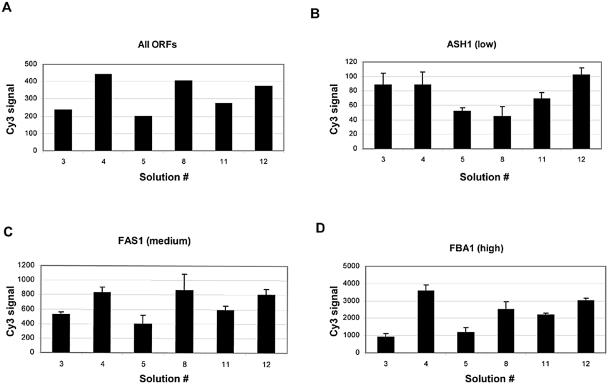
To further validate our observations, we arrayed the entire set of 24 ORFs with solutions 3, 4, 5, 8, 11 and 12 (solutions 5 and 8 were included as these solutions produced morphologically homogenous spots), in quadruplicate, onto two different slides and assayed spot quality using the criteria described above. As in the first experiment, all of the DNAs were printed at the same concentration for each solution using an individual pin for each ORF. As a result, each patch or block of spots represented one ORF spotted with the six different solutions by a single pin. Therefore, any differences between replicates could not be attributed to systematic differences contributed by the robot. Figure 2 shows example images of slides either stained with SYBR Green II (Fig. 2A) or after hybridization (Fig. 2B), with the layout of each patch shown on the right (Fig. 2C). Given the limitations of using a single pin to deposit one ORF it is difficult to ascribe absolute mRNA amounts for each ORF in both samples, however, we detected a specific pattern of hybridization, which differed greatly across the set of 24 ORFs (Fig. 2B) and was in agreement with previously reported data (17). Figure 3A shows the average Cy3 signal intensity (the mean of all background-subtracted median pixel intensities) for each solution. On the whole, we observed a larger signal from spots printed in formamide (solutions 4, 8 and 12) as compared to spots printed with the same detergent and DMSO (solutions 3, 5 and 11, respectively; Fig. 3A). While the average signal was not significantly different between solutions 4, 8 and 12, we did observe differences on a gene-by-gene basis (Fig. 3B–D). Altogether, these data show that the spots printed with solution 12 consistently yielded a high and reproducible signal.
Figure 2.
Representative images of 24 ORFs and negative control DNA spotted in quadruplicate using six different spotting solutions. (A) An image of a slide containing 24 patches each with 24 spots in which each patch is composed of a single ORF stained with SYBR Green II (SG). A replicate slide after hybridization with fluorescently labeled cDNA is shown in (B). Each patch contains one ORF printed in quadruplicate (R1–R4) and with six different solutions (S1–S6). The organization of each ORF patch is shown by the indicated patch (green box) in (C). SYBR Green II stains all of the DNA that was spotted, whereas the image after hybridization shows the specificity of the interaction between the labeled target and the spotted probe.
Figure 3.
Bar graphs of the overall background-subtracted Cy3 signal measured for each solution from two replicate slides following hybridization. (A) The average Cy3 signal obtained for all ORFs (n = 192) for each solution on two different slides is shown in this bar graph. Since the levels of expression of the ORFs vary to such a large extent error bars are not included. As a measure of reproducibility, the data (average background signals with error bars, n = 8) for three ORFs representing a low, medium and high expressing gene are shown in (B), (C) and (D), respectively.
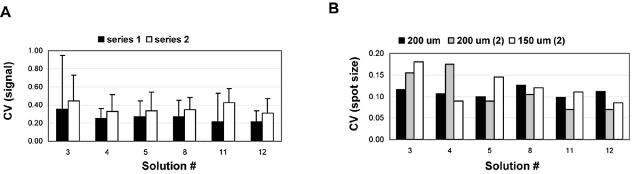
Moreover, we found that 1% CHAPS (solutions 11 and 12) provided the highest homogeneity of pixel intensity distribution (Fig. 4, Series 1). Performing multiple Student’s t-tests we found that solutions 11 and 12 were statistically indistinguishable, while the differences between solution 12 and the other four solutions were highly significant (P < 0.002). Although the average CV for solutions 11 and 12 was lower than that measured from the other solutions, this level of signal homogeneity was found to be more reproducible with spots printed with solution 12 compared to solution 11.
Figure 4.
Summary of the CVs for the pixel intensities and spot sizes and the variation with time and different pin sizes. (A) Average CVs of the signal intensities derived from 24 ORFs spotted in the indicated solution on two different days (series 1 and 2). Each bar represents the average CV measured from four replicates of each ORF and on two replicate slides (n = 192). The standard deviation CVs for each mean CV are represented by error bars. Series 1 corresponds to data obtained from freshly spotted slides and Series 2 corresponds to data obtained from slides spotted after storing the source plates at 4°C for 4 months. (B) CVs of spot sizes corresponding to Series 1 from (A) (black), to Series 2 (gray) and to spots printed with smaller pins (150 µm) at the same time as Series 2 (white).
In order to test the effect of time and pin size we stored the 384-well source plates for 4 months at 4°C, arrayed two replicate slides using the same format as previously described for two pin sizes (200 and 150 µm diameter pin tip) and hybridized them with freshly prepared, fluorescently labeled cDNA derived from a new batch of RNA. We observed that for all spotting solutions, storage over time under the above conditions affected the average quality of the spot morphology (Fig. 4A, Series 2). Nevertheless, we found that solution 12 produced the most reproducibly homogeneous spots. We also observed that solutions 4 and 12 yielded the most uniform spot sizes using a smaller pin size (Fig. 4B). Altogether, these data show that solution 12 yields spots whose individual pixel intensities and size are more homogeneous and reproducible than the other spotting solutions and is more robust using different spotting parameters.
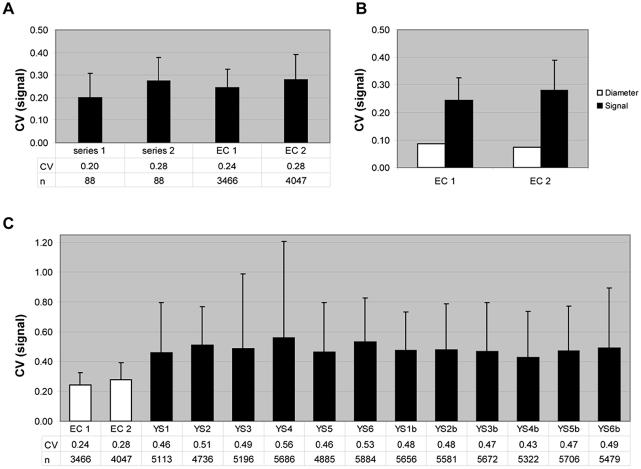
In order to assess the ability of this solution to preserve spot morphology on a larger scale, we applied the same criteria to replicate slides arrayed with amplified cDNAs corresponding to the entire genome of E.coli. The cDNAs were printed with solution 12 using 150 µm pins. A summary of the data is shown in Figure 5. We observed low CV values for both spot size and spot pixel intensities similar to what were calculated using the 24 yeast ORFs set. In addition, we found that these CV values were significantly lower than those measured from widely used commercially available slides (Fig. 5C). Using the same hybridization protocol we measured the homogeneity of spot morphology using commercially available arrays containing ORFs corresponding to the entire S.cerevisiae genome. While information concerning the spotting solution and robot used to generate the slides are not publicly available, these slides have an identical surface chemistry to the slides used in our experiments. Although we observed low background values and consistent spot sizes across all 12 arrays (not indicated), we observed a high level of variation in spot morphology (Fig. 5C, indicated by the higher average and standard deviation CV values for all 12 yeast slides compared to E.coli slides).
Figure 5.
(A) Recapitulation of average and standard deviation for the CVs of the signal intensity shown in Figure 4A for solution 12 compared to two independent hybridizations with slides printed with cDNAs corresponding to the entire genome of E.coli (EC1 and EC2, respectively). (B) The CVs of measured spot diameter (white) and pixel intensities (average and standard deviation of CVs of the Cy3 pixel intensity shown in black) from EC1 and EC2. (C) Average and standard deviation of the CVs for the pixel intensities measured from EC1 and EC2 compared to commercially available yeast whole-genome slides. Twelve independent yeast slides were analyzed representing 12 different yeast cultures from six different strains, where YS1–YS6 corresponds to the different strains and the duplicate cultures of the same strain are denoted with a b. n refers to the number of spots included in the analysis.
In microarray studies, one relies on the measurement of up to tens of thousands of spots on one slide multiplied by the number of slides in a given study. The accuracy of these measurements is of paramount importance in order to extract meaningful biological information. It has been well documented that systematic and random errors reduce the accuracy of such measurements. In this study, we have tested a variety of spotting solutions in order to increase spot homogeneity and reproducibility with the intent to increase accuracy and ultimately to decrease systematic errors. We found that solutions containing detergent additives above their CMCs produced more homogeneous spot morphologies while size reproducibility depended more on the combination of detergent and solvent. We found that the zwitterionic detergent CHAPS (1%) in combination with formamide (50%), both of which are readily available, reduced the variation by increasing the homogeneity and reproducibility of spot morphology of replicate spot measurements across multiple slides compared not only to widely used and published spotting solutions, but also to a well-known commercially available pre-spotted slide. Considering that only slight differences were observed between solutions 11 and 12 and given the teratogenic properties of formamide, some users may prefer to use CHAPS (1%) in DMSO (50%). While CHAPS does not possess a formal charge between pH 1 and 13, it appears, nevertheless, capable of significant electrostatic interactions due to the presence of a strong dipole moment. This characteristic, coupled with its ability to reduce the surface tension, is advantageous to better stabilize the heavily charged environment of spotted DNA (a negative charge contributed by the sugar–phosphate backbone of the DNA coupled with the positive charge of the slide surface during the drying process), leading to more homogeneous spots. In conclusion, optimization of spotting solution composition by the addition of detergent additives may significantly increase the reproducibility of cDNA microarrays and lead to improved quantitative analyses.
Acknowledgments
ACKNOWLEDGEMENT
The authors would like to thank Dr Frederic Boccard (Centre de Génétique Moléculaire, Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) for providing the entire 4288 ORF E.coli DNA set and for his help in generating the accompanying microarrays and images used for the analysis.
REFERENCES
- 1.Velculescu V.E., Zhang,L., Vogelstein,B. and Kinzler,K.W. (1995) Serial analysis of gene expression. Science, 270, 484–487. [DOI] [PubMed] [Google Scholar]
- 2.Wodicka L., Dong,H., Mittmann,M., Ho,M.H. and Lockhart,D.J. (1997) Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat. Biotechnol., 15, 1359–1367. [DOI] [PubMed] [Google Scholar]
- 3.DeRisi J.L., Iyer,V.R. and Brown,P.O. (1997) Exploring the metabolic and genetic control of gene expression on a genomic scale. Science, 278, 680–686. [DOI] [PubMed] [Google Scholar]
- 4.Schena M., Shalon,D., Davis,R.W. and Brown,P.O. (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science, 270, 467–470. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart D.J., Dong,H., Byrne,M.C., Follettie,M.T., Gallo,M.V., Chee,M.S., Mittmann,M., Wang,C., Kobayashi,M., Horton,H. et al. (1996) Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat. Biotechnol., 14, 1675–1680. [DOI] [PubMed] [Google Scholar]
- 6.Schulze A. and Downward,J. (2001) Navigating gene expression using microarrays—a technology review. Nature Cell Biol., 3, E190–E195. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y.H., Dudoit,S., Luu,P., Lin,D.M., Peng,V., Ngai,J. and Speed,T.P. (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res., 30, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak J.P., Sladek,R. and Hudson,T.J. (2002) Characterization of variability in large-scale gene expression data: implications for study design. Genomics, 79, 104–113. [DOI] [PubMed] [Google Scholar]
- 9.Kerr M.K., Martin,M. and Churchill,G.A. (2000) Analysis of variance for gene expression microarray data. J. Comput. Biol., 7, 819–837. [DOI] [PubMed] [Google Scholar]
- 10.Churchill G.A. (2002) Fundamentals of experimental design for cDNA microarrays. Nature Genet., 32, 490–495. [DOI] [PubMed] [Google Scholar]
- 11.Hegde P., Qi,R., Abernathy,K., Gay,C., Dharap,S., Gaspard,R., Hughes,J.E., Snesrud,E., Lee,N. and Quackenbush,J. (2000) A concise guide to cDNA microarray analysis. Biotechniques, 29, 548–550, 552,–544, 556passim. [DOI] [PubMed] [Google Scholar]
- 12.Shalon D., Smith,S.J. and Brown,P.O. (1996) A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res., 6, 639–645. [DOI] [PubMed] [Google Scholar]
- 13.Diehl F., Grahlmann,S., Beier,M. and Hoheisel,J.D. (2001) Manufacturing DNA microarrays of high spot homogeneity and reduced background signal. Nucleic Acids Res., 29, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennetzen J.L. and Hall,B.D. (1982) Codon selection in yeast. J. Biol. Chem., 257, 3026–3031. [PubMed] [Google Scholar]
- 15.Cherry J.M., Ball,C., Weng,S., Juvik,G., Schmidt,R., Adler,C., Dunn,B., Dwight,S., Riles,L., Mortimer,R.K. et al. (1997) Genetic and physical maps of Saccharomyces cerevisiae. Nature, 387, 67–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Sharp P.M. and Li,W.H. (1986) Codon usage in regulatory genes in Escherichia coli does not reflect selection for ‘rare’ codons. Nucleic Acids Res., 14, 7737–7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holstege F.C., Jennings,E.G., Wyrick,J.J., Lee,T.I., Hengartner,C.J., Green,M.R., Golub,T.R., Lander,E.S. and Young,R.A. (1998) Dissecting the regulatory circuitry of a eukaryotic genome. Cell, 95, 717–728. [DOI] [PubMed] [Google Scholar]
- 18.Racki W.J., Becam,A.M., Nasr,F. and Herbert,C.J. (2000) Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J., 19, 4524–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espeli O. and Boccard,F. (1997) In vivo cleavage of Escherichia coli BIME-2 repeats by DNA gyrase: genetic characterization of the target and identification of the cut site. Mol. Microbiol., 26, 767–777. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein J.A., Khodursky,A.B., Lin,P.H., Lin-Chao,S. and Cohen,S.N. (2002) Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc. Natl Acad. Sci. USA, 99, 9697–9702. [DOI] [PMC free article] [PubMed] [Google Scholar]