Abstract
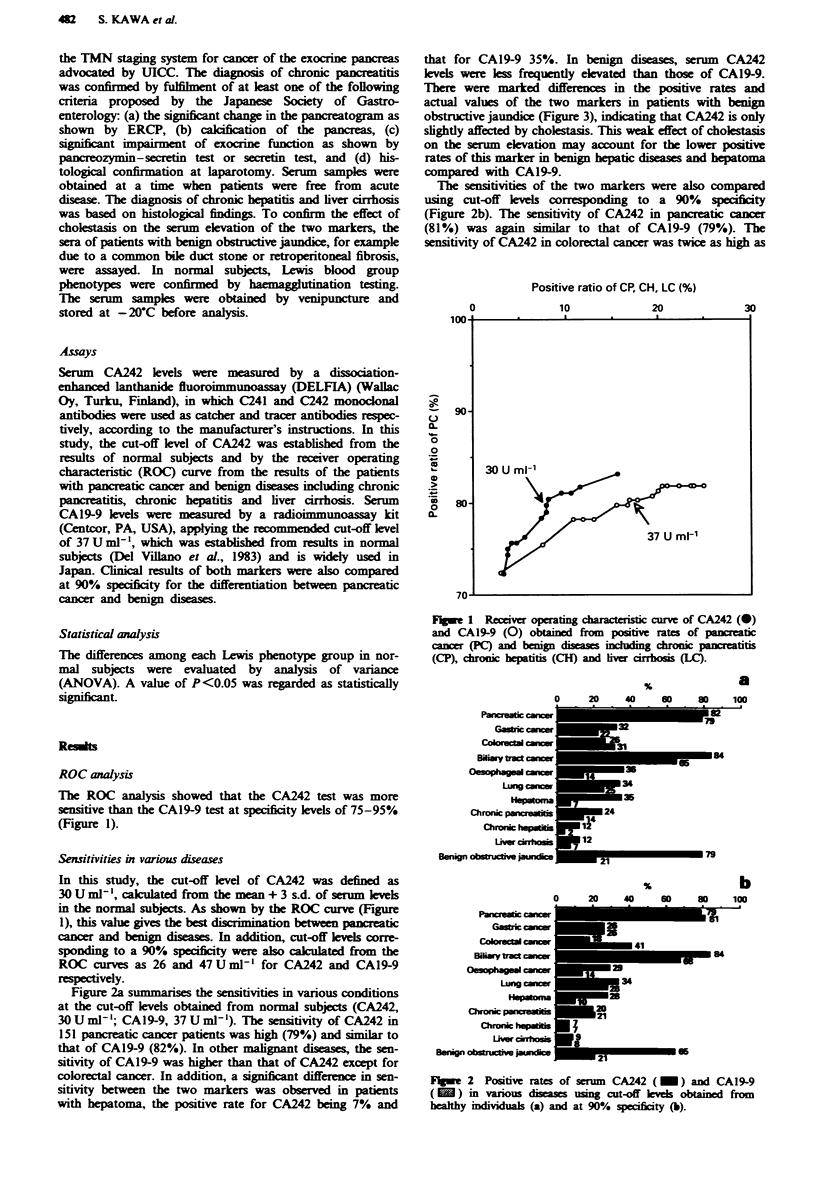
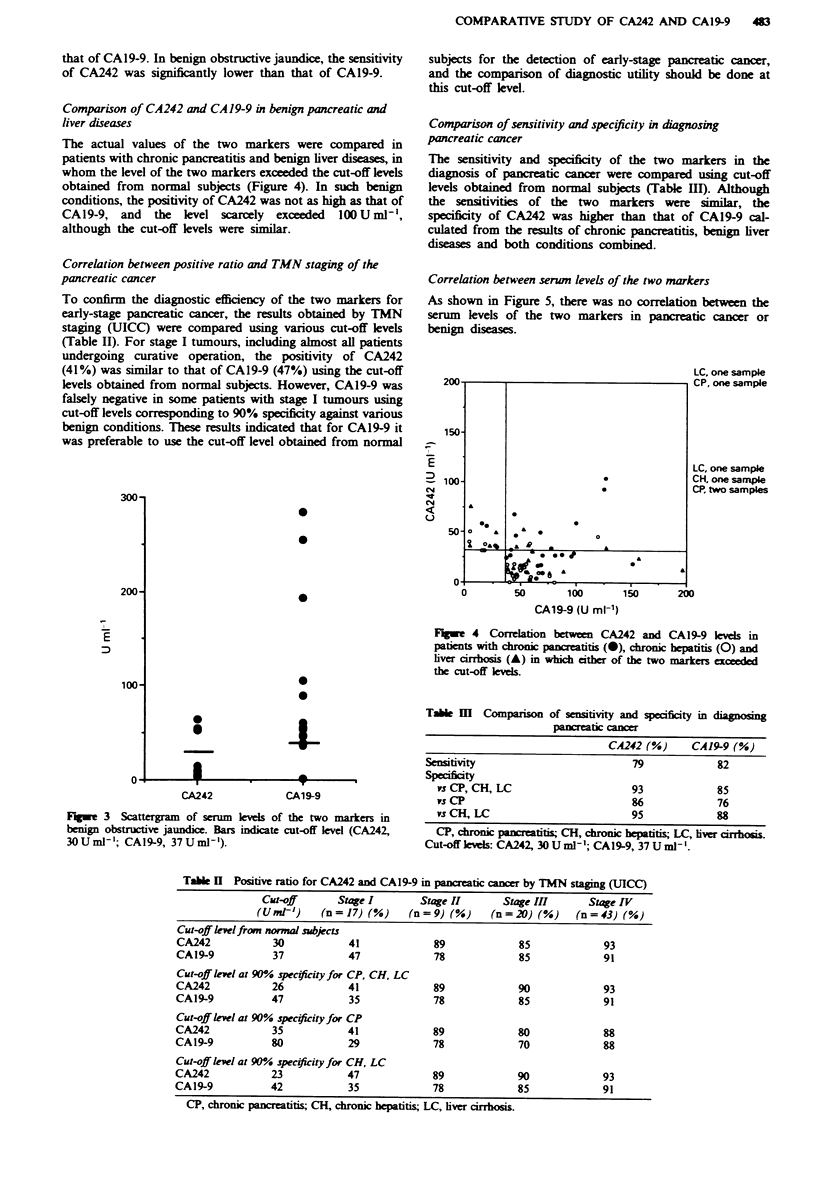
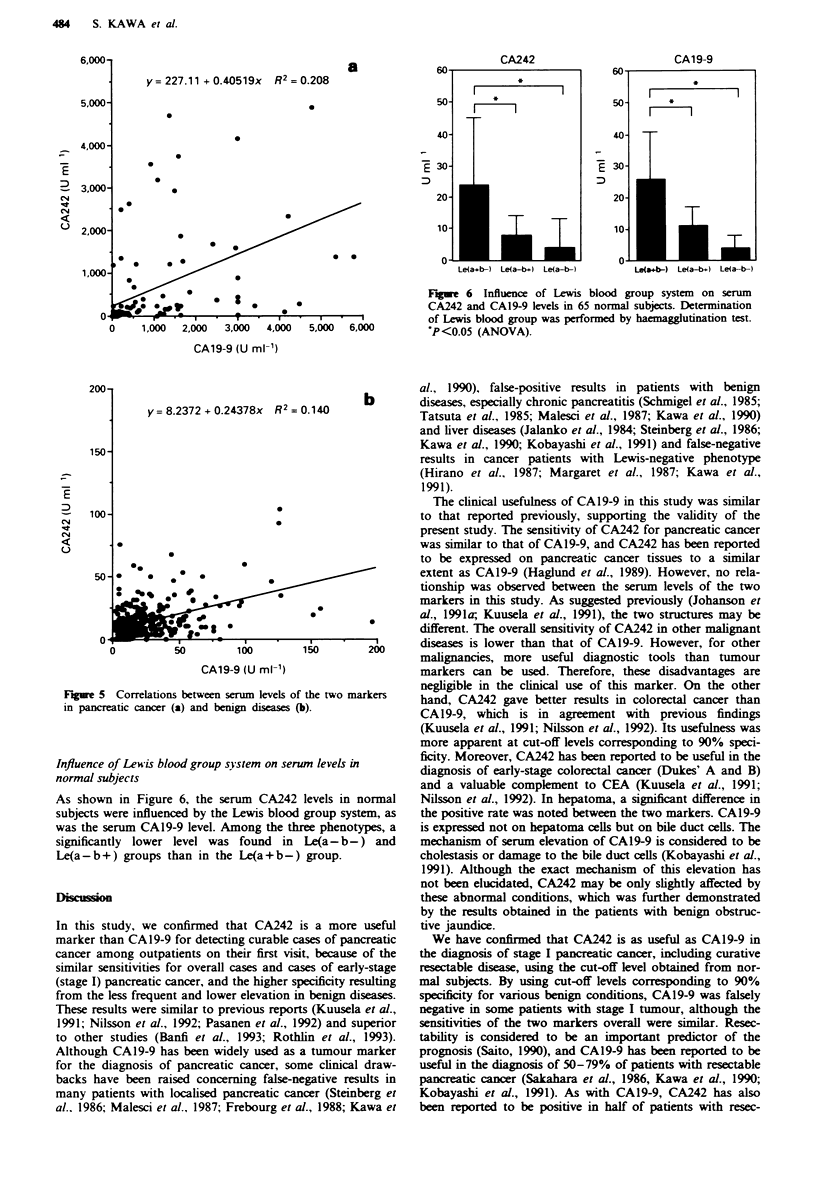
A comparative study of a new tumour marker, CA242, and CA19-9 was conducted with special reference to their diagnostic usefulness in pancreatic cancer. CA242 showed sensitivity similar to that of CA19-9 for overall cases and early cases (stage I tumour) of pancreatic cancer. For other malignancies, the positive rates of CA242 were lower than those of CA19-9 except for colorectal cancer. An important characteristics of CA242 was that it was only slightly and infrequently elevated in the sera of patients with benign diseases such as chronic pancreatitis, chronic hepatitis and liver cirrhosis. This characteristic was more apparent in the patients with benign obstructive jaundice, indicating that the serum level of this marker was scarcely affected by cholestasis. Using cut-off levels corresponding to a 90% specificity, the clinical results obtained with CA242 in the diagnosis of pancreatic cancer were similar to those obtained with CA19-9, except that CA19-9 was falsely negative in some patients with early-stage pancreatic cancer. These findings suggest the usefulness of this marker for screening pancreatic cancer in patients on their first hospital visit. However, CA242 was found to be influenced by the Lewis blood group system. This unfavourable result is attributed to the C241 catcher antibody of this assay system, which has almost the same epitope specificity as the C50 and the NS19-9 monoclonal antibodies. In conclusion, CA242 is superior to CA19-9 in diagnosing pancreatic cancer by virtue of its higher specificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J. W., Verstynen C., Bosman F. T., Hilgers J., Steplewski Z. Distribution of monoclonal antibody-defined monosialoganglioside in normal and cancerous human tissues: an immunoperoxidase study. Hybridoma. 1983;2(2):219–229. doi: 10.1089/hyb.1983.2.219. [DOI] [PubMed] [Google Scholar]
- Banfi G., Zerbi A., Pastori S., Parolini D., Di Carlo V., Bonini P. Behavior of tumor markers CA19.9, CA195, CAM43, CA242, and TPS in the diagnosis and follow-up of pancreatic cancer. Clin Chem. 1993 Mar;39(3):420–423. [PubMed] [Google Scholar]
- Del Villano B. C., Brennan S., Brock P., Bucher C., Liu V., McClure M., Rake B., Space S., Westrick B., Schoemaker H. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983 Mar;29(3):549–552. [PubMed] [Google Scholar]
- Frebourg T., Bercoff E., Manchon N., Senant J., Basuyau J. P., Breton P., Janvresse A., Brunelle P., Bourreille J. The evaluation of CA 19-9 antigen level in the early detection of pancreatic cancer. A prospective study of 866 patients. Cancer. 1988 Dec 1;62(11):2287–2290. doi: 10.1002/1097-0142(19881201)62:11<2287::aid-cncr2820621103>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Haglund C., Lindgren J., Roberts P. J., Kuusela P., Nordling S. Tissue expression of the tumour associated antigen CA242 in benign and malignant pancreatic lesions. A comparison with CA 50 and CA 19-9. Br J Cancer. 1989 Dec;60(6):845–851. doi: 10.1038/bjc.1989.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Kawa S., Oguchi H., Kobayashi T., Yonekura H., Ogata H., Homma T. Loss of Lewis antigen expression on erythrocytes in some cancer patients with high serum CA19-9 levels. J Natl Cancer Inst. 1987 Dec;79(6):1261–1268. [PubMed] [Google Scholar]
- Homma T., Tsuchiya R. The study of the mass screening of persons without symptoms and of the screening of outpatients with gastrointestinal complaints or icterus for pancreatic cancer in Japan, using CA19-9 and elastase-1 or ultrasonography. Int J Pancreatol. 1991 Summer;9:119–124. doi: 10.1007/BF02925587. [DOI] [PubMed] [Google Scholar]
- Jalanko H., Kuusela P., Roberts P., Sipponen P., Haglund C. A., Mäkelä O. Comparison of a new tumour marker, CA 19-9, with alpha-fetoprotein and carcinoembryonic antigen in patients with upper gastrointestinal diseases. J Clin Pathol. 1984 Feb;37(2):218–222. doi: 10.1136/jcp.37.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C., Nilsson O., Baeckström D., Jansson E. L., Lindholm L. Novel epitopes on the CA50-carrying antigen: chemical and immunochemical studies. Tumour Biol. 1991;12(3):159–170. doi: 10.1159/000217701. [DOI] [PubMed] [Google Scholar]
- Johansson C., Nilsson O., Lindholm L. Comparison of serological expression of different epitopes on the CA50-carrying antigen CanAg. Int J Cancer. 1991 Jul 9;48(5):757–763. doi: 10.1002/ijc.2910480521. [DOI] [PubMed] [Google Scholar]
- Kawa S., Oguchi H., Kobayashi T., Tokoo M., Furuta S., Kanai M., Homma T. Elevated serum levels of Dupan-2 in pancreatic cancer patients negative for Lewis blood group phenotype. Br J Cancer. 1991 Nov;64(5):899–902. doi: 10.1038/bjc.1991.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Kawa S., Tokoo M., Oguchi H., Kiyosawa K., Furuta S., Kanai M., Homma T. Comparative study of CA-50 (time-resolved fluoroimmunoassay), Span-1, and CA19-9 in the diagnosis of pancreatic cancer. Scand J Gastroenterol. 1991 Jul;26(7):787–797. doi: 10.3109/00365529108998600. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Haglund C., Roberts P. J. Comparison of a new tumour marker CA 242 with CA 19-9, CA 50 and carcinoembryonic antigen (CEA) in digestive tract diseases. Br J Cancer. 1991 Apr;63(4):636–640. doi: 10.1038/bjc.1991.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malesci A., Tommasini M. A., Bonato C., Bocchia P., Bersani M., Zerbi A., Beretta E., Di Carlo V. Determination of CA 19-9 antigen in serum and pancreatic juice for differential diagnosis of pancreatic adenocarcinoma from chronic pancreatitis. Gastroenterology. 1987 Jan;92(1):60–67. doi: 10.1016/0016-5085(87)90840-7. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Johansson C., Glimelius B., Persson B., Nørgaard-Pedersen B., Andrén-Sandberg A., Lindholm L. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992 Feb;65(2):215–221. doi: 10.1038/bjc.1992.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Månsson J. E., Lindholm L., Holmgren J., Svennerholm L. Sialosyllactotetraosylceramide, a novel ganglioside antigen detected in human carcinomas by a monoclonal antibody. FEBS Lett. 1985 Mar 25;182(2):398–402. doi: 10.1016/0014-5793(85)80341-0. [DOI] [PubMed] [Google Scholar]
- Pasanen P. A., Eskelinen M., Partanen K., Pikkarainen P., Penttilä I., Alhava E. Clinical evaluation of a new serum tumour marker CA 242 in pancreatic carcinoma. Br J Cancer. 1992 May;65(5):731–734. doi: 10.1038/bjc.1992.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röthlin M. A., Joller H., Largiadèr F. CA 242 is a new tumor marker for pancreatic cancer. Cancer. 1993 Feb 1;71(3):701–707. doi: 10.1002/1097-0142(19930201)71:3<701::aid-cncr2820710308>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sakahara H., Endo K., Nakajima K., Nakashima T., Koizumi M., Ohta H., Hidaka A., Kohno S., Nakano Y., Naito A. Serum CA 19-9 concentrations and computed tomography findings in patients with pancreatic carcinoma. Cancer. 1986 Apr 1;57(7):1324–1326. doi: 10.1002/1097-0142(19860401)57:7<1324::aid-cncr2820570712>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Schmiegel W. H., Kreiker C., Eberl W., Arndt R., Classen M., Greten H., Jessen K., Kalthoff H., Soehendra N., Thiele H. G. Monoclonal antibody defines CA 19-9 in pancreatic juices and sera. Gut. 1985 May;26(5):456–460. doi: 10.1136/gut.26.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta M., Yamamura H., Iishi H., Ichii M., Noguchi S., Yamamoto R., Okuda S. Values of CA 19-9 in the serum, pure pancreatic juice, and aspirated pancreatic material in the diagnosis of malignant pancreatic tumor. Cancer. 1985 Dec 1;56(11):2669–2673. doi: 10.1002/1097-0142(19851201)56:11<2669::aid-cncr2820561124>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Tempero M. A., Uchida E., Takasaki H., Burnett D. A., Steplewski Z., Pour P. M. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987 Oct 15;47(20):5501–5503. [PubMed] [Google Scholar]



