Abstract
Objective
To examine whether blood pressure in early pregnancy and its rise in second half of gestation are associated with spontaneous preterm birth in healthy, normotensive, nulliparous women.
Methods
We included 5,167 women with singleton gestation who participated in the World Health Organization Calcium Supplementation for the Prevention of Preeclampsia Trial. Systolic, diastolic, mean arterial blood pressure and pulse pressure at baseline (12 – 19 weeks of gestation) and mid 3rd trimester (30 – 34 weeks) were calculated. Rise in blood pressure was the difference between the mid 3rd trimester and baseline. Preterm birth was defined as early preterm (< 34 completed weeks) and late preterm birth (34 – 36 weeks).
Results
Women experiencing early or late preterm birth had over 10 mmHg and 3 mmHg higher rise, respectively, in systolic, diastolic and mean arterial blood pressure than women delivering at term. A rise in systolic pressure over 30 mmHg or diastolic pressure over 15 mmHg was associated with a statistically significant 2 – 3-fold increase in risk of spontaneous preterm birth.
Conclusion
An excessive rise in either systolic or diastolic blood pressures from early pregnancy to mid 3rd trimester is associated with spontaneous preterm birth in a dose-response pattern.
Keywords: blood pressure, preeclampsia, preterm birth
INTRODUCTION
Preterm birth remains a major contributor to adverse perinatal outcomes. Hypertension in pregnancy, preeclampsia in particular, is one of the main causes of preterm birth, mostly due to medically indicated deliveries, frequently associated with poor outcomes.1,2 Interestingly, Ananth et al. reported an almost 2-fold increase in risk of spontaneous preterm labor in women with preeclampsia, comparing to normotensive women.1 Overall, etiology of spontaneous preterm birth is often unclear3 and the dynamics of blood pressure during pregnancy in relation to spontaneous preterm birth has not been well studied. We examined whether blood pressure level in early pregnancy and the magnitude of its rise between the 1st and 3rd trimesters are associated with preterm birth in healthy, normotensive, nulliparous women.
MATERIALS AND METHODS
This is a secondary analysis using data from the World Health Organization Calcium Supplementation for the Prevention of Preeclampsia Trial. The trial was approved by the Institutional Review Boards of all participating institutions. Detailed description of the trial is provided elsewhere.4 Briefly, a total of 8,325 normotensive nulliparous women were enrolled before 20 weeks of gestation from clinics serving women with low dietary calcium intake (<600 mg/day) in 6 countries (Argentina, Egypt, India, Peru, South Africa and Vietnam) between 2001 and 2003. Women were randomized to receive either 1.5 g calcium/day supplement or placebo tablets throughout pregnancy. Demographic and clinical information was recorded at enrollment.
Blood pressure was measured at each antenatal care visit with a mercury sphygmomanometer twice (the average was used) at 3-minute intervals, with the subject seated for at least 5 minutes before measurement with the cuff positioned at the level of the heart on the right arm. Great efforts were made on training, certification and retraining of clinic staff every 3 months to obtain the blood pressure measures as accurate as possible.5
There was no difference between the treatment and the control groups at baseline on maternal demographic and clinical characteristics, gestational age at enrollment, mean systolic and diastolic blood pressure at randomization.4 The trial found that calcium supplementation did not significantly reduce the risk of overall preeclampsia. The mean systolic and diastolic blood pressure levels at 30 – 34 weeks were identical at 108 and 64 mmHg, respectively, for the treatment and the control groups (Student t-tests on systolic and diastolic pressure, both p > 0.05). Thus, we assumed that calcium supplementation did not influence mean blood pressure and combined the two groups for the current analysis. However, calcium supplementation was found to mitigate the severity of the disease, including preterm delivery.4 Therefore, we controlled for calcium supplementation in the multivariable analysis as a potential confounder.
Baseline blood pressure was designated as the average pressure recorded between 12 and 19 weeks of gestation and mid 3rd trimester blood pressure as the average of any measurement between 30 and 34 weeks of gestation. Rise (or fall) in blood pressure was calculated as the difference between the mid 3rd trimester and baseline pressures, and pulse pressure, difference between systolic and diastolic measurements.
Gestational age at entry was determined with the “best obstetric estimate”, which included the last menstrual period, uterine size and ultrasound examination if required by the attending physician.4 At each prenatal visit and admission to delivery, date and estimated gestational age were recorded. To ensure accuracy of gestational age, we calculated a gestational age at delivery as follows: Gestational age at enrollment + (delivery date – enrollment date)/7, then took the integer and compared the calculated gestational age at delivery with the recorded gestational age at delivery as completed weeks. Any discrepancy greater than one week resulted in exclusion of the subject due to the possibility that the gestational age was incorrect. Type of labor onset was categorized as spontaneous or medically indicated (induced or prelabor cesarean section).
There were a total of 8,002 nulliparous women with a singleton pregnancy who were normotensive at enrollment and had complete delivery information. 6,547 (82%) women had an accurate gestational age. 9 had postterm deliveries (>42 weeks) and were excluded, as were 1,323 women with any of the following conditions: complaint of any health problems at the first visit, stillbirth in present pregnancy, or delivered a newborn with any congenital malformations. These exclusions left 5,167 subjects for analysis.
Because blood pressure measurements were all normally distributed, descriptive statistics were expressed as mean and standard deviations. Analysis of variance and chi-square test were used for continuous and categorical variables, respectively. We used multiple linear regression and Cox proportional hazard model to control for potential confounders for continuous and time-to-event outcomes, respectively. The potential confounders included maternal age, education, maternal body mass index, gestational age at admission, smoking status, race, gravidity and calcium treatment. Data analysis was done in SAS (Statistical Analysis System version 9.0. Cary, NC).
RESULTS
The incidence of preterm birth was 7.6% in this cohort of healthy, normotensive, nulliparous women with a singleton gestation. Table I presents the baseline characteristics of women according to gestational age at delivery: term (≥ 37 weeks), late preterm (34 – 36 weeks) and early preterm birth (< 34 weeks). The average gestational age at admission was 15 weeks. The vast majority of women were primigravida and very few smoked. Women with preterm birth were significantly younger as compared with women delivering at term. African and Middle Eastern women tended to have a higher rate of preterm birth while South East Asian women had a substantially lower rate. A higher percentage of preterm births were delivered by elective cesarean section.
Table I.
Baseline characteristics of the subjects for the analysis of blood pressure during pregnancy and preterm birth. All deliveries are considered.
| Term Birth (≥ 37 wks) (N = 4804) | Late Preterm Birth (34 – 36 wks) (N = 289) | Early Preterm Birth (<34 wks) (N = 74) | p value* | ||
|---|---|---|---|---|---|
| Age | 23.3 (4.4) | 22.1 (4.2) | 21.7 (3.8) | <.0001 | |
| Education (years in school) | 10 (3.3) | 9.9 (3.8) | 9.8 (3.5) | .60 | |
| Body mass index, kg/m² | 21.5 (4.0) | 21.7 (4.2) | 22.2 (4.3) | .16 | |
| Gestational age at admission | 15.8 (3.2) | 15.1 (3.3) | 16.3 (2.7) | .0007 | |
| Gravidity | 1 | 4165 (92.8%) | 262 (5.8%) | 64 (1.4%) | .15 |
| 2+ | 639 (94.5%) | 27 (4.0%) | 10 (1.5%) | ||
| Smoker | Yes | 198 (94.3%) | 9 (4.3%) | 3 (1.4%) | .71 |
| No | 4603 (93.0%) | 279 (5.6%) | 70 (1.4%) | ||
| Race | European | 1083 (93.6%) | 61 (5.3%) | 13 (1.1%) | <.0001 |
| Indian | 1387 (91.8%) | 108 (7.1%) | 16 (1.1%) | ||
| African | 602 (85.5%) | 72 (10.2%) | 30 (4.3%) | ||
| Middle Eastern | 289 (88.4%) | 29 (8.9%) | 9 (2.7%) | ||
| South East Asian | 1443 (98.3%) | 19 (1.3%) | 6 (0.4%) | ||
| Calcium treatment | Yes | 2422 (93.4%) | 143 (5.5%) | 29 (1.1%) | 0.15 |
| No | 2382 (92.6%) | 146 (5.6%) | 45 (1.8%) | ||
| Onset of labor | Spontaneous | 4199 (93.8%) | 229 (5.1%) | 51 (1.1%) | <.0001 |
| Induced | 457 (91.0%) | 35 (7.0%) | 10 (2.0%) | ||
| Elective caesarean delivery | 144 (79.5%) | 24 (13.3%) | 13 (7.2%) | ||
Analysis of variance test for continuous variables and Chi-square test for categorical variables.
Table II presents mean blood pressure at baseline and rise from baseline to 30 – 34 weeks according to preterm/term status. Although mean systolic and diastolic blood pressure and mean arterial pressure were similar among these groups in early pregnancy, the baseline pulse pressure was significantly higher among women destined to have an early preterm birth. Furthermore, the rise in systolic, diastolic and mean arterial pressure between early gestation and mid 3rd trimester values was significantly greater in early and late preterm births in a dose-response pattern. These findings were confirmed in multiple linear regression analysis adjusting for several maternal characteristics and calcium supplementation status (Table III). Women who had early or late preterm birth had over 10 and 3 mmHg higher rise, respectively, in systolic, diastolic and mean arterial blood pressure than women with term births.
Table II.
Unadjusted mean blood pressure (SD) in mmHg during pregnancy according to gestational age at delivery. All deliveries are considered.
| Blood pressure at baseline (12 – 19 wks) | Term Birth (≥ 37 wks) (N = 4804) | Late Preterm Birth (34 – 36 wks) (N = 289) | Early Preterm Birth (< 34 wks) (N = 74) | p value* |
|---|---|---|---|---|
| Diastolic | 61 (9) | 61 (9) | 59 (10) | .06 |
| Systolic | 106 (10) | 107 (11) | 108 (10) | .07 |
| Mean arterial | 76 (8) | 76 (9) | 75 (9) | .50 |
| Pulse pressure | 44 (9) | 45 (10) | 49 (11) | <.0001 |
| Increase from baseline to 30–34 wks | (N = 4430) | (N = 269) | (N = 57) | |
| Diastolic | 2.9 (9.4) | 7.4 (11.9) | 14.6 (18.0) | <.0001 |
| Systolic | 2.5 (10.6) | 5.7 (12.0) | 15.0 (24.0) | <.0001 |
| Mean arterial | 2.8 (8.5) | 6.8 (11.0) | 14.7 (19.1) | <.0001 |
| Pulse pressure | −0.5 (10.5) | −1.7 (10.1) | 0.4 (13.8) | .12 |
Analysis of variance tests.
Table III.
Adjusted change in blood pressure during pregnancy according to gestational age at delivery. All deliveries are considered.
| Blood pressure at baseline (12 – 19 wks) | Term Birth (≥ 37 wks) | Late Preterm Birth (34 – 36 wks) Beta-coeff.* (95%CI) | Early Preterm Birth (< 34 wks) Beta-coeff.* (95%CI) |
|---|---|---|---|
| Diastolic | Reference | 0.84 (−0.09, 1.77) | −0.81 (−2.61, 0.99) |
| Systolic | Reference | 0.52 (−0.59, 1.62) | 1.43 (−0.71, 3.58) |
| Mean arterial | Reference | 0.72 (−0.15, 1.58) | −0.05 (−1.74, 1.63) |
| Pulse pressure | Reference | −0.36 (−1.38, 0.66) | 2.21 (0.24, 4.19) |
| Increase from baseline to 30–34 wks | |||
| Diastolic | Reference | 3.6 (2.5, 4.8) | 10.8 (8.3, 13.4) |
| Systolic | Reference | 3.1 (1.7, 4.4) | 12.1 (9.1, 15.0) |
| Mean arterial | Reference | 3.5 (2.4, 4.6) | 11.2 (8.8, 13.5) |
| Pulse pressure | Reference | −0.6 (−1.8, 0.7) | 1.2 (−1.5, 4.0) |
Multiple linear regression adjusting for maternal age, education, maternal body mass index, gestational age at admission, smoking status, race, gravidity and calcium supplementation. CI: confidence interval.
Because preeclampsia or severe hypertension often leads to labor induction or elective cesarean delivery, we restricted our analysis to women with spontaneous labor. Table IV shows that the rise in systolic, diastolic and arterial pressure was still significantly greater among spontaneous early and late preterm births with a clear dose-response pattern, though the magnitude of rise was somewhat mitigated. When we further restricted analysis to women manifesting neither gestational hypertension nor preeclampsia, the pattern and statistical significance remained the same but the magnitude was reduced modestly (results not shown).
Table IV.
Adjusted change in blood pressure during pregnancy and spontaneous preterm birth*. Only spontaneous initiation of labor is considered.
| Blood pressure at baseline (12 – 19 wks) | Term Birth (≥ 37 wks) | Late Preterm Birth (34 – 36 wks) Beta-coeff.* (95%CI) | Early Preterm Birth (< 34 wks) Beta-coeff.* (95%CI) |
|---|---|---|---|
| Diastolic | Reference | 0.35 (−0.67, 1.38) | −2.08 (−4.21, 0.05) |
| Systolic | Reference | −0.17 (−1.40, 1.06) | 0.49 (−2.06, 3.04) |
| Mean arterial | Reference | 0.16 (−0.80, 1.12) | −1.26 (−3.25, 0.74) |
| Pulse pressure | Reference | −0.58 (−1.71, 0.56) | 2.54 (0.18, 4.89) |
| Increase from baseline to 30–34 wks | |||
| Diastolic | Reference | 3.0 (1.7, 4.3) | 5.4 (2.4, 8.3) |
| Systolic | Reference | 2.5 (1.0, 4.0) | 5.8 (2.4, 9.1) |
| Mean arterial | Reference | 2.8 (1.7, 4.0) | 5.4 (2.7, 8.1) |
| Pulse pressure | Reference | −0.5 (−1.9, 0.9) | 0.4 (−2.9, 3.6) |
Multiple linear regression adjusting for maternal age, education, maternal body mass index, gestational age at admission, smoking status, race, gravidity and calcium supplementation. CI: confidence interval.
Figure 1 depicts the relationship between rise in blood pressure and incidence of spontaneous preterm birth (< 37 weeks). A greater rise in systolic and diastolic pressure was associated with a statistically significant 2 – 3-fold increase in spontaneous preterm birth after having adjusted for potential confounders and calcium supplementation.
Figure 1.
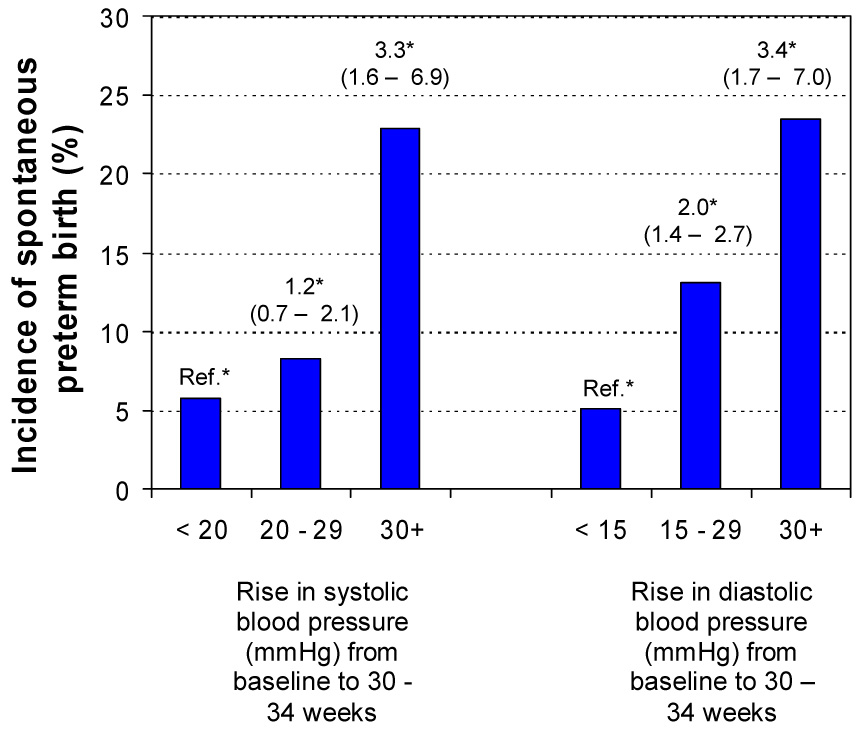
Incidence of spontaneous preterm birth according to rise in systolic and diastolic blood pressure from 12 – 19 weeks to 30 – 34 weeks of gestation. *Hazard ratio and 95% confidence interval using Cox proportional hazard model adjusting for maternal age, education, maternal body mass index, gestational age at admission, smoking status, race, gravidity and calcium supplementation.
COMMENT
Preeclampsia has been associated with preterm birth in recent epidemiologic studies6, mostly because of early medical intervention for maternal and/or fetal indications. Our analysis of a carefully conducted large prospective study4 shows that long before clinical manifestation of hypertension, high pulse pressure in early pregnancy is already a risk factor for spontaneous early preterm birth. Furthermore, a greater rise in blood pressure from early pregnancy to mid 3rd trimester is associated with spontaneous preterm birth in a dose-response pattern, even in women who remain normotensive. These findings are consistent with finding from a previous study, where preeclampsia was associated with an increased risk of moderately preterm births due to spontaneous labor (RR = 1.9, 95% CI: 1.3 – 2.8).1
Our data do not permit conclusions on causality. We speculate that several mechanisms may explain our findings. Trophoblastic invasion and spiral artery remodeling early in pregnancy transforms a low-volume high-resistance circulation to a high-volume low-resistance circulation within the uterus.7 Uterine artery Doppler studies clearly demonstrate that the resistance index decreases substantially from the non-pregnant state to early pregnancy, the decline continuing until 30 weeks of gestation.8 Prospective studies showed that an abnormally high uterine artery pulsatility index9 and notching10 in the second trimester is associated with a significantly increased risk of preterm birth, supporting our finding on maternal pulse pressure. Olofsson et al.11 linked high uterine artery pulsatility index with defective development or lack of physiological vessel changes in placental bed spiral arteries. Thus, it is possible that rise in pulse pressure is a compensatory response to help provide a sufficient blood supply to a defective placenta. Abnormal placentation, by itself, may also predispose these pregnancies to a higher risk of spontaneous preterm birth.12 A common placental link may, therefore, explain why there is an association between high pulse pressure and uterine artery pulsatility index and an increased risk of spontaneous preterm birth.
We also observed that increments in systolic and diastolic blood pressure from early pregnancy to the mid 3rd trimester associated with spontaneous preterm birth had a clear dose-response pattern. This association could also be explained by a common placental mechanism. Naeye13 found a strong association between preeclampsia, placental alterations suggestive of low uteroplacental blood flow and spontaneous preterm birth. Salafia et al.14 showed that mean maternal arterial blood pressure at admission to labor was positively associated with the presence/extent of uteroplacental vascular lesions and with lesions of intraplacental vaso-occlusion. Again, abnormal placentation may predispose these women to a higher risk of spontaneous preterm birth.
There are other factors related to the vasoconstrictive state of preeclamptic women that may contribute to spontaneous preterm birth. Preeclampsia is characterized by increased levels of circulating vasocontrictors including endothelin-1, thromboxane, norepinephrine, as well as an increase in sympathetic tone, while levels of vasodilators such as nitric oxide and prostacycline are decreased.15 Such changes also affect uterine myometrium contractility.16–18 For example, Maltaris et al.16 in an in vitro study perfused swine uteri with endothelin-1 and sera from preeclamptic, normal pregnant and non-pregnant women. Intrauterine pressure was significantly higher in uteri perfused by sera from preeclamptic women compared with sera from normotensive pregnant women. Uterine hypercontractility in preeclampsia has long been documented in human.19 Thus, elevated levels of vasoconstrictors combined with reduced levels of vasodilators may contribute to spontaneous preterm birth even in the absence of preeclampsia.
In summary, our results indicate that high pulse pressure in early pregnancy is a risk factor for spontaneous early preterm birth. Also, excessive increases in blood pressure from early pregnancy to mid 3rd trimester are associated independently with spontaneous preterm birth in a dose-response pattern.
ACKNOWLEDGMENT
J. Zhang., W. Sun. and K. F. Yu were supported by the National Institutes of Health Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ananth CV, Savitz DA, Luther ER, Bowes WA., Jr Preeclampsia and preterm birth subtypes in Nova Scotia, 1986 to 1992. Am J Perinatol. 1997;14:17–23. doi: 10.1055/s-2007-994090. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Abalos E, Carroli G, et al. Heterogeneity of perinatal outcomes in the preterm delivery syndrome. Obstet Gynecol. 2004;104:78–87. doi: 10.1097/01.AOG.0000130837.57743.7b. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL. The management of preterm labor. Obstet Gynecol. 2002;100:1020–1037. doi: 10.1016/s0029-7844(02)02212-3. [DOI] [PubMed] [Google Scholar]
- 4.Villar J, Abdel-Aleen H, Merialdi M, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194:639–649. doi: 10.1016/j.ajog.2006.01.068. [DOI] [PubMed] [Google Scholar]
- 5.Villar J, Say L, Shennan A, Lindheimer M, Duley L, Conde-Agudelo A, et al. Methodological and technical issues related to the prediction, diagnostic and treatment of preeclampsia and eclampsia. Int J Obstet Gynecol. 2004;85:S28–S41. doi: 10.1016/j.ijgo.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, Campodonico L, Al-Mazrou Y, Lindheimer M, Kramer M. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM. Pregnancy-related hypertension. In: Creasy RK, Resnk R, Iams JD, editors. Maternal-Fetal Medicine: principles and practice. 5th edition. Philadelphia, PA: Saunders; 2004. pp. 859–893. [Google Scholar]
- 8.Tekay A, Campbell S. Doppler ultrasonography in obstetrics. In: Callen PW, editor. Ultrasonography in Obstetrics and Gynecology. 4th edition. Philadelphia, PA: Saunders; 2000. pp. 677–689. [Google Scholar]
- 9.Aardema MW, Saro MCS, Lander M, de Wolf BTHM, Oosterhof H, Aarnoudse JG. Second trimester Doppler ultrasound screening of the uterine arteries differentiates between subsequent normal and poor outcomes of hypertensive pregnancy: two different pathophysiological entities? Clin Sci. 2004;106:377–382. doi: 10.1042/CS20030385. [DOI] [PubMed] [Google Scholar]
- 10.El-Hamedi A, Shillito J, Simpson NA, Walker JJ. A prospective analysis of the role of uterine artery Doppler waveform notching in the assessment of at-risk pregnancies. Hypertens Pregnancy. 2005;24:137–145. doi: 10.1081/PRG-200059857. [DOI] [PubMed] [Google Scholar]
- 11.Olofsson P, Laurini RN, Marsal K. A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation. Eur J Obstet Gynecol Reprod Biol. 1993;49:161–168. doi: 10.1016/0028-2243(93)90265-e. [DOI] [PubMed] [Google Scholar]
- 12.Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 13.Naeye RL. Pregnancy hypertension, placental evidences of low uteroplacental blood flow, and spontaneous premature delivery. Hum Pathol. 1989;20:441–444. doi: 10.1016/0046-8177(89)90008-7. [DOI] [PubMed] [Google Scholar]
- 14.Salafia CM, Ghidini A, Lopez-Zeno JA, Pezzullo JC. Uteroplacental pathology and maternal arterial mean blood pressure in spontaneous prematurity. J Soc Gynecol Invest. 1998;5:68–71. doi: 10.1016/S1071-5576(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RN, Roberts JM. Endothelial cell dysfunction. In: Lindheimer MD, Roberts JM, Cunningham FG, editors. Chesley’s Hypertensive Disorders in Pregnancy. 2nd edition. Stamford, CT: Appleton & Lange; 1999. pp. 395–429. [Google Scholar]
- 16.Maltaris T, Scalera F, Schlembach D, et al. Increased uterine arterial pressure and contractility of perfused swine uterus after treatment with serum from preeclamptic women and endothelin-1. Clin Sci. 2005;109:209–215. doi: 10.1042/CS20040340. [DOI] [PubMed] [Google Scholar]
- 17.Maul H, Longo M, Saade GR, Garfield RE. Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharmaceutical Design. 2003;9:359–380. doi: 10.2174/1381612033391784. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelmsson L, Wikland M, Wiqvist N. PGH2, TxA2 and PGI2 have potent and differentiated actions on human uterine contractility. Prostaglandins. 1981;21:277–286. doi: 10.1016/0090-6980(81)90145-3. [DOI] [PubMed] [Google Scholar]
- 19.Cobo E. Uterine hypercontractility in toxemia of pregnancy. I. Its prolonged therapeutic control. Am J Obstet Gynecol. 1964;90:505–510. doi: 10.1016/0002-9378(64)90809-9. [DOI] [PubMed] [Google Scholar]


