Abstract
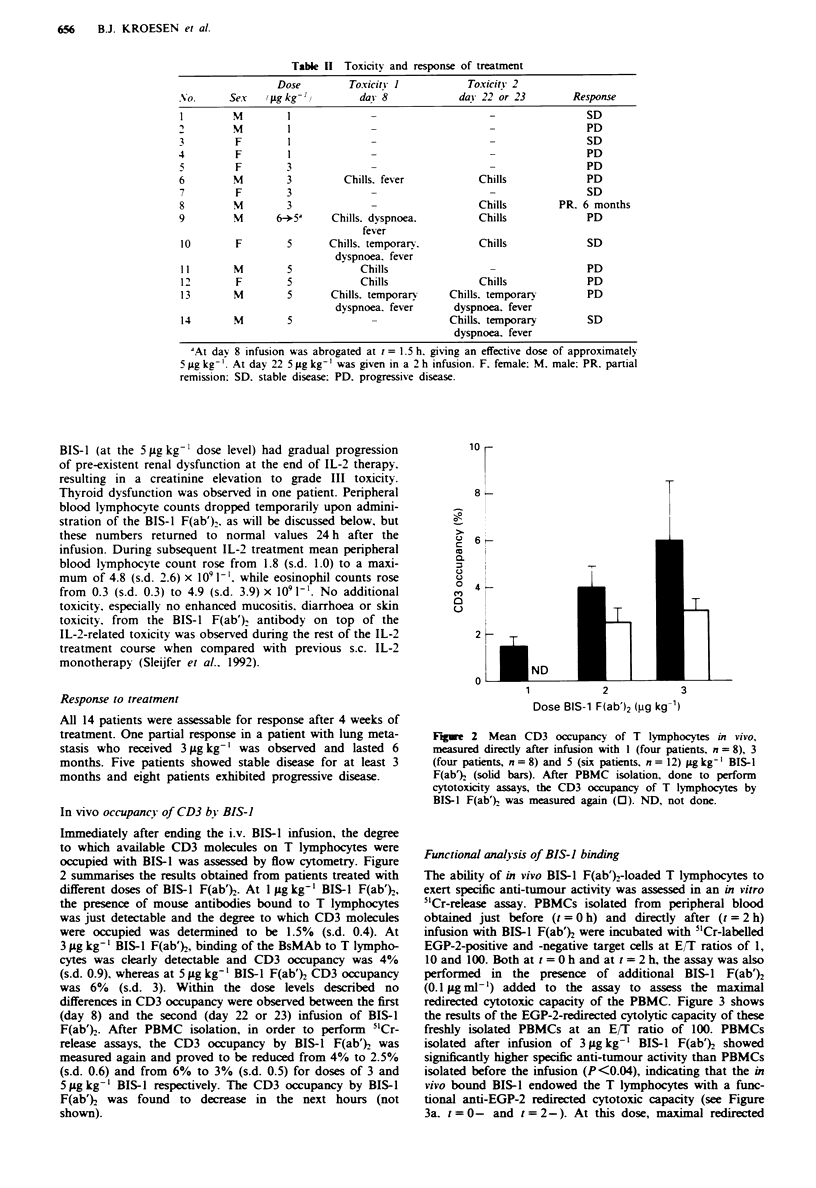
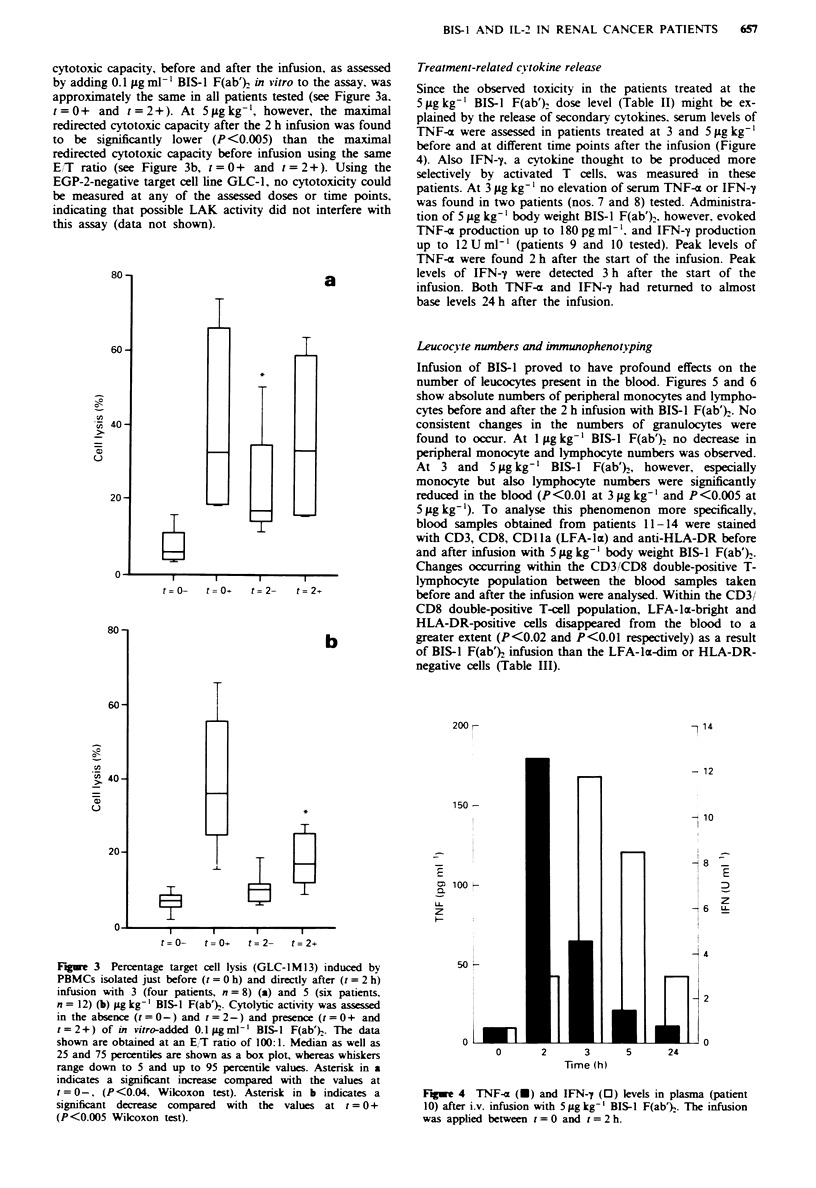
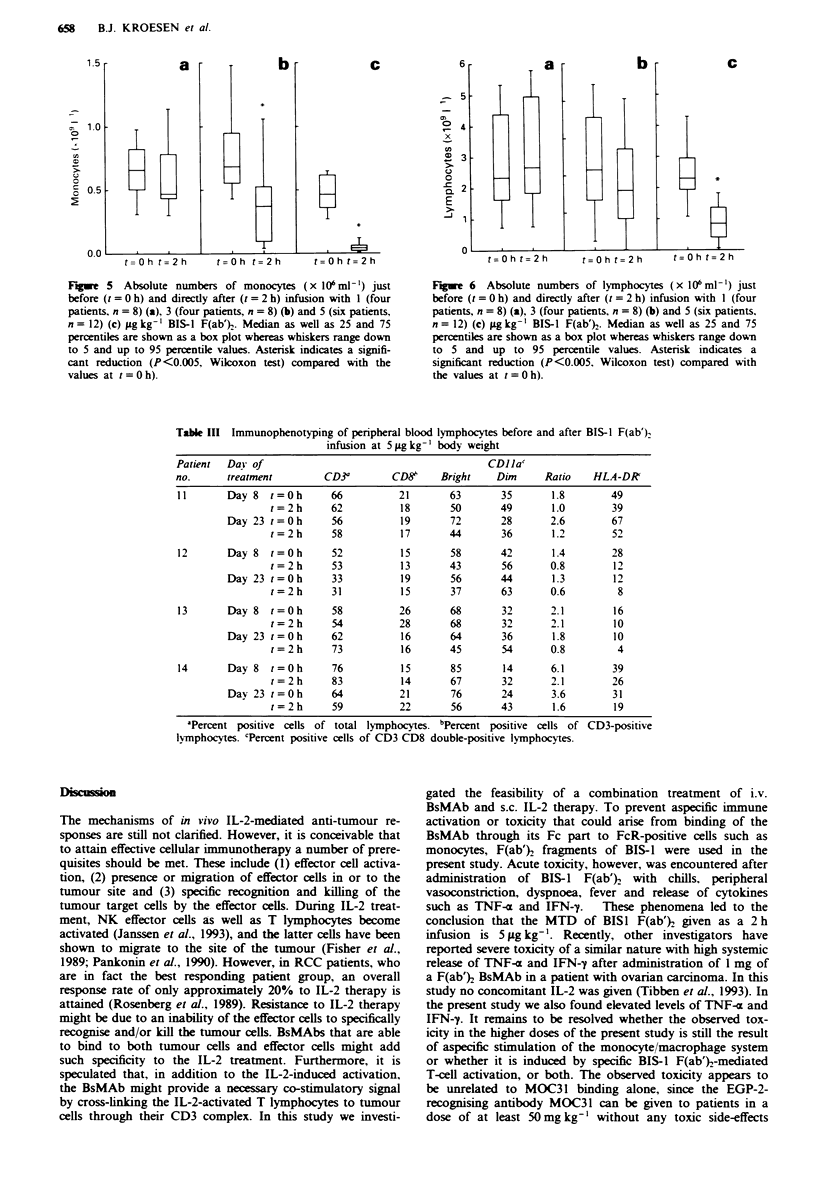
In a phase I trial the toxicity and immunomodulatory effects of combined treatment with intravenous (i.v.) bispecific monoclonal antibody BIS-1 and subcutaneous (s.c.) interleukin 2 (IL-2) was studied in renal cell cancer patients. BIS-1 combines a specificity against CD3 on T lymphocytes with a specificity against a 40 kDa pancarcinoma-associated antigen, EGP-2. Patients received BIS-1 F(ab')2 fragments intravenously at doses of 1, 3 and 5 micrograms kg-1 body weight during a concomitantly given standard s.c. IL-2 treatment. For each dose, four patients were treated with a 2 h BIS-1 infusion in the second and fourth week of IL-2 therapy. Acute BIS-1 F(ab')2-related toxicity with symptoms of chills, peripheral vasoconstriction and temporary dyspnoea was observed in 2/4 and 5/5 patients at the 3 and 5 micrograms kg-1 dose level respectively. The maximum tolerated dose (MTD) of BIS-1 F(ab')2 was 5 micrograms kg-1. Elevated plasma levels of tumour necrosis factor alpha (TNF-alpha) and interferon gamma (IFN-gamma) were detected at the MTD. Flow cytometric analysis showed a dose-dependent binding of BIS-1 F(ab')2 to circulating T lymphocytes. Peripheral blood mononuclear cells (PBMCs), isolated after treatment with 3 and 5 micrograms kg-1 BIS-1, showed increased specific cytolytic capacity against EGP-2+ tumour cells as tested in an ex vivo performed assay. Maximal killing capacity of the PBMCs, as assessed by adding excess BIS-1 to the assay, was shown to be decreased after BIS-1 infusion at 5 micrograms kg-1 BIS-1 F(ab')2. A BIS-1 F(ab')2 dose-dependent disappearance of circulating mononuclear cells from the peripheral blood was observed. Within the circulating CD3+ CD8+ lymphocyte population. LFA-1 alpha-bright and HLA-DR+ T-cell numbers decreased preferentially. It is concluded that i.v. BIS-1 F(ab')2, when combined with s.c. IL-2, has a MTD of 5 micrograms kg-1. The treatment endows the T lymphocytes with a specific anti-EGP-2-directed cytotoxic potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Geller R. L., Nelson P. J., Panzer S., Gromo G., Benfield M. R., Inverardi L., Podack E. R., Witson J. C., Houchins J. P. A "minimal signal-stepwise activation" analysis of functional maturation of T lymphocytes. Immunol Rev. 1989 Oct;111:35–57. doi: 10.1111/j.1600-065x.1989.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Blank-Voorthuis C. J., Braakman E., Ronteltap C. P., Tilly B. C., Sturm E., Warnaar S. O., Bolhuis R. L. Clustered CD3/TCR complexes do not transduce activation signals after bispecific monoclonal antibody-triggered lysis by cytotoxic T lymphocytes via CD3. J Immunol. 1993 Sep 15;151(6):2904–2914. [PubMed] [Google Scholar]
- Bolhuis R. L., Lamers C. H., Goey S. H., Eggermont A. M., Trimbos J. B., Stoter G., Lanzavecchia A., di Re E., Miotti S., Raspagliesi F. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study. Int J Cancer Suppl. 1992;7:78–81. [PubMed] [Google Scholar]
- Bolhuis R. L., Sturm E., Braakman E. T cell targeting in cancer therapy. Cancer Immunol Immunother. 1991;34(1):1–8. doi: 10.1007/BF01741317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman E., Goedegebuure P. S., Vreugdenhil R. J., Segal D. M., Shaw S., Bolhuis R. L. ICAM- melanoma cells are relatively resistant to CD3-mediated T-cell lysis. Int J Cancer. 1990 Sep 15;46(3):475–480. doi: 10.1002/ijc.2910460325. [DOI] [PubMed] [Google Scholar]
- Buter J., Janssen R. A., Martens A., Sleijfer D. T., de Leij L., Mulder N. H. Phase I/II study of low-dose intravenous OKT3 and subcutaneous interleukin-2 in metastatic cancer. Eur J Cancer. 1993;29A(15):2108–2113. doi: 10.1016/0959-8049(93)90044-g. [DOI] [PubMed] [Google Scholar]
- De Jong R., Brouwer M., Rebel V. I., Van Seventer G. A., Miedema F., Van Lier R. A. Generation of alloreactive cytolytic T lymphocytes by immobilized anti-CD3 monoclonal antibodies. Analysis of requirements for human cytolytic T-lymphocyte differentiation. Immunology. 1990 Jul;70(3):357–364. [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Prigione I., Miotti S., Ciccone E., Cantoni C., Chen Q., Colnaghi M. I., Moretta L. Bispecific monoclonal antibodies directed to CD16 and to a tumor-associated antigen induce target-cell lysis by resting NK cells and by a subset of NK clones. Int J Cancer. 1991 May 10;48(2):227–233. doi: 10.1002/ijc.2910480213. [DOI] [PubMed] [Google Scholar]
- Fisher B., Packard B. S., Read E. J., Carrasquillo J. A., Carter C. S., Topalian S. L., Yang J. C., Yolles P., Larson S. M., Rosenberg S. A. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989 Feb;7(2):250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Greaves M. W. Early cellular and molecular events in inflamed skin: an integrated concept. J Dermatol Sci. 1992 Jan;3(1):1–5. doi: 10.1016/0923-1811(92)90002-s. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L., Theander T. G., Abdulhadi N. H., Abu-Zeid Y. A., Bayoumi R. A., Jensen J. B. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. Eur J Immunol. 1991 May;21(5):1249–1253. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- Janssen R. A., Buter J., Straatsma E., Heijn A. A., Sleijfer D. T., de Vries E. G., Mulder N. H., The T. H., de Leij L. HLA-Dr-expressing CD8bright cells are only temporarily present in the circulation during subcutaneous recombinant interleukin-2 therapy in renal cell carcinoma patients. Cancer Immunol Immunother. 1993;36(3):198–204. doi: 10.1007/BF01741092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L., Huntoon C., Donohue J., Leibson P. J., Bander N. H., Ghose T., Luner S. J., Vessella R., McKean D. J. Heteroconjugate antibody-directed killing of autologous human renal carcinoma cells by in vitro-activated lymphocytes. J Immunol. 1990 May 15;144(10):4060–4067. [PubMed] [Google Scholar]
- Kroesen B. J., ter Haar A., Spakman H., Willemse P., Sleijfer D. T., de Vries E. G., Mulder N. H., Berendsen H. H., Limburg P. C., The T. H. Local antitumour treatment in carcinoma patients with bispecific-monoclonal-antibody-redirected T cells. Cancer Immunol Immunother. 1993 Nov;37(6):400–407. doi: 10.1007/BF01526797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanzanica D., Garrido M. A., Neblock D. S., Daddona P. E., Andrew S. M., Zurawski V. R., Jr, Segal D. M., Wunderlich J. R. Human T-lymphocytes targeted against an established human ovarian carcinoma with a bispecific F(ab')2 antibody prolong host survival in a murine xenograft model. Cancer Res. 1991 Oct 15;51(20):5716–5721. [PubMed] [Google Scholar]
- Morimoto C., Rudd C. E., Letvin N. L., Schlossman S. F. A novel epitope of the LFA-1 antigen which can distinguish killer effector and suppressor cells in human CD8 cells. Nature. 1987 Dec 3;330(6147):479–482. doi: 10.1038/330479a0. [DOI] [PubMed] [Google Scholar]
- Nitta T., Sato K., Yagita H., Okumura K., Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990 Feb 17;335(8686):368–371. doi: 10.1016/0140-6736(90)90205-j. [DOI] [PubMed] [Google Scholar]
- Rivoltini L., Cattoretti G., Arienti F., Mastroianni A., Melani C., Colombo M. P., Parmiani G. The high lysability by LAK cells of colon-carcinoma cells resistant to doxorubicin is associated with a high expression of ICAM-1, LFA-3, NCA and a less-differentiated phenotype. Int J Cancer. 1991 Mar 12;47(5):746–754. doi: 10.1002/ijc.2910470521. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonigg H., Wilders-Truschnig M., Loibner H., Plot R., Rot A., Kuss I., Werner G., Stöger H., Wrann M., Herlyn D. Immune response to tumor antigens in a patient with colorectal cancer after immunization with anti-idiotype antibody. Clin Immunol Immunopathol. 1992 Dec;65(3):271–277. doi: 10.1016/0090-1229(92)90157-j. [DOI] [PubMed] [Google Scholar]
- Schwab R., Crow M. K., Russo C., Weksler M. E. Requirements for T cell activation by OKT3 monoclonal antibody: role of modulation of T3 molecules and interleukin 1. J Immunol. 1985 Sep;135(3):1714–1718. [PubMed] [Google Scholar]
- Segal D. M., Qian J. H., Andrew S. M., Titus J. A., Mezzanzanica D., Garrido M. A., Wunderlich J. R. Cytokine release by peripheral blood lymphocytes targeted with bispecific antibodies, and its role in blocking tumor growth. Ann N Y Acad Sci. 1991 Dec 30;636:288–294. doi: 10.1111/j.1749-6632.1991.tb33459.x. [DOI] [PubMed] [Google Scholar]
- Sleijfer D. T., Janssen R. A., Buter J., de Vries E. G., Willemse P. H., Mulder N. H. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on an outpatient basis. J Clin Oncol. 1992 Jul;10(7):1119–1123. doi: 10.1200/JCO.1992.10.7.1119. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Chang T. H., Herlyn M., Koprowski H. Release of monoclonal antibody-defined antigens by human colorectal carcinoma and melanoma cells. Cancer Res. 1981 Jul;41(7):2723–2727. [PubMed] [Google Scholar]
- Stötter H., Wiebke E. A., Tomita S., Belldegrun A., Topalian S., Rosenberg S. A., Lotze M. T. Cytokines alter target cell susceptibility to lysis. II. Evaluation of tumor infiltrating lymphocytes. J Immunol. 1989 Mar 1;142(5):1767–1773. [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]
- Tibben J. G., Boerman O. C., Claessens R. A., Corstens F. H., van Deuren M., de Mulder P. H., van der Meer J. W., Keijser K. G., Massuger L. F. Cytokine release in an ovarian carcinoma patient following intravenous administration of bispecific antibody OC/TR F(ab')2. J Natl Cancer Inst. 1993 Jun 16;85(12):1003–1004. doi: 10.1093/jnci/85.12.1003. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N. M., Reisfeld R. A., Walker L. E. Antigens associated with a human lung adenocarcinoma defined by monoclonal antibodies. Cancer Res. 1984 Feb;44(2):681–687. [PubMed] [Google Scholar]
- Vogetseder W., Feichtinger H., Schulz T. F., Schwaeble W., Tabaczewski P., Mitterer M., Böck G., Marth C., Dapunt O., Mikuz G. Expression of 7F7-antigen, a human adhesion molecule identical to intercellular adhesion molecule-1 (ICAM-1) in human carcinomas and their stromal fibroblasts. Int J Cancer. 1989 May 15;43(5):768–773. doi: 10.1002/ijc.2910430504. [DOI] [PubMed] [Google Scholar]
- Webb D. S., Mostowski H. S., Gerrard T. L. Cytokine-induced enhancement of ICAM-1 expression results in increased vulnerability of tumor cells to monocyte-mediated lysis. J Immunol. 1991 May 15;146(10):3682–3686. [PubMed] [Google Scholar]
- Weiner G. J., Hillstrom J. R. Bispecific anti-idiotype/anti-CD3 antibody therapy of murine B cell lymphoma. J Immunol. 1991 Dec 1;147(11):4035–4044. [PubMed] [Google Scholar]
- Weiner L. M., Holmes M., Adams G. P., LaCreta F., Watts P., Garcia de Palazzo I. A human tumor xenograft model of therapy with a bispecific monoclonal antibody targeting c-erbB-2 and CD16. Cancer Res. 1993 Jan 1;53(1):94–100. [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- Zola H., Neoh S. H., Mantzioris B. X., Webster J., Loughnan M. S. Detection by immunofluorescence of surface molecules present in low copy numbers. High sensitivity staining and calibration of flow cytometer. J Immunol Methods. 1990 Dec 31;135(1-2):247–255. doi: 10.1016/0022-1759(90)90278-4. [DOI] [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]


