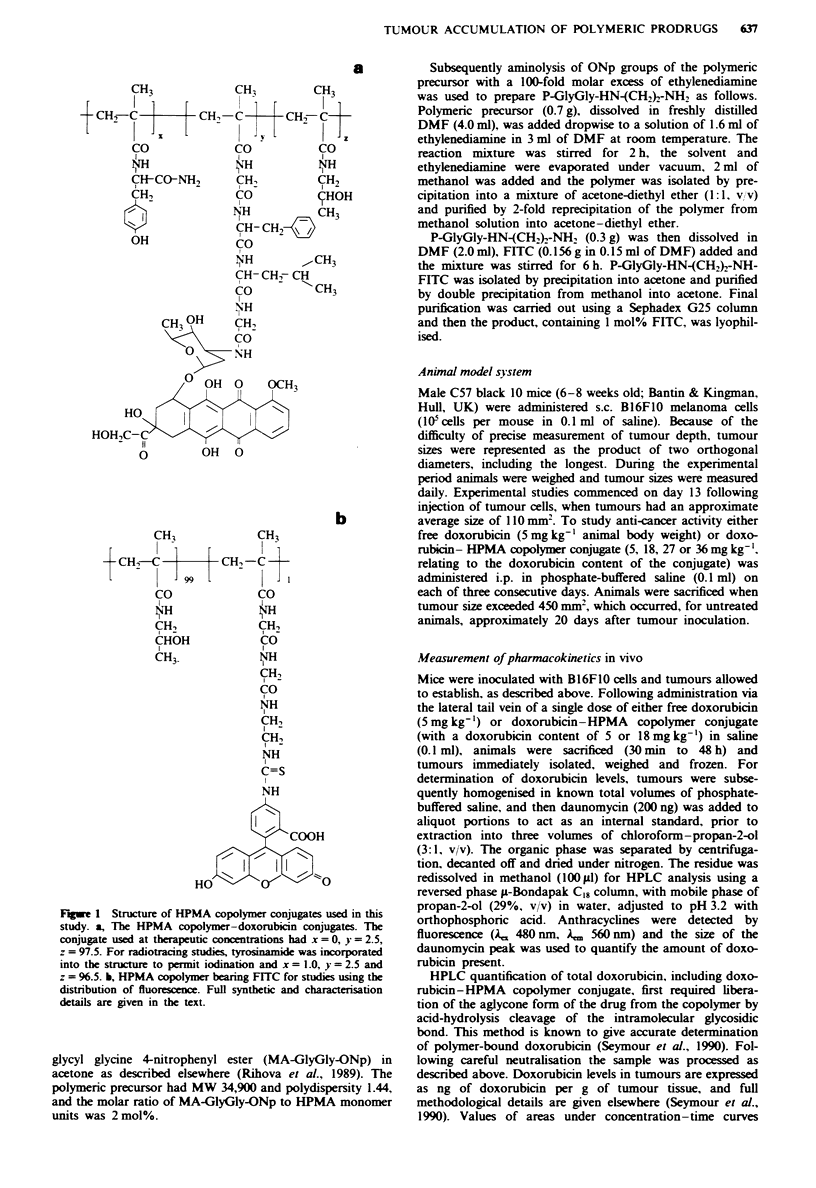
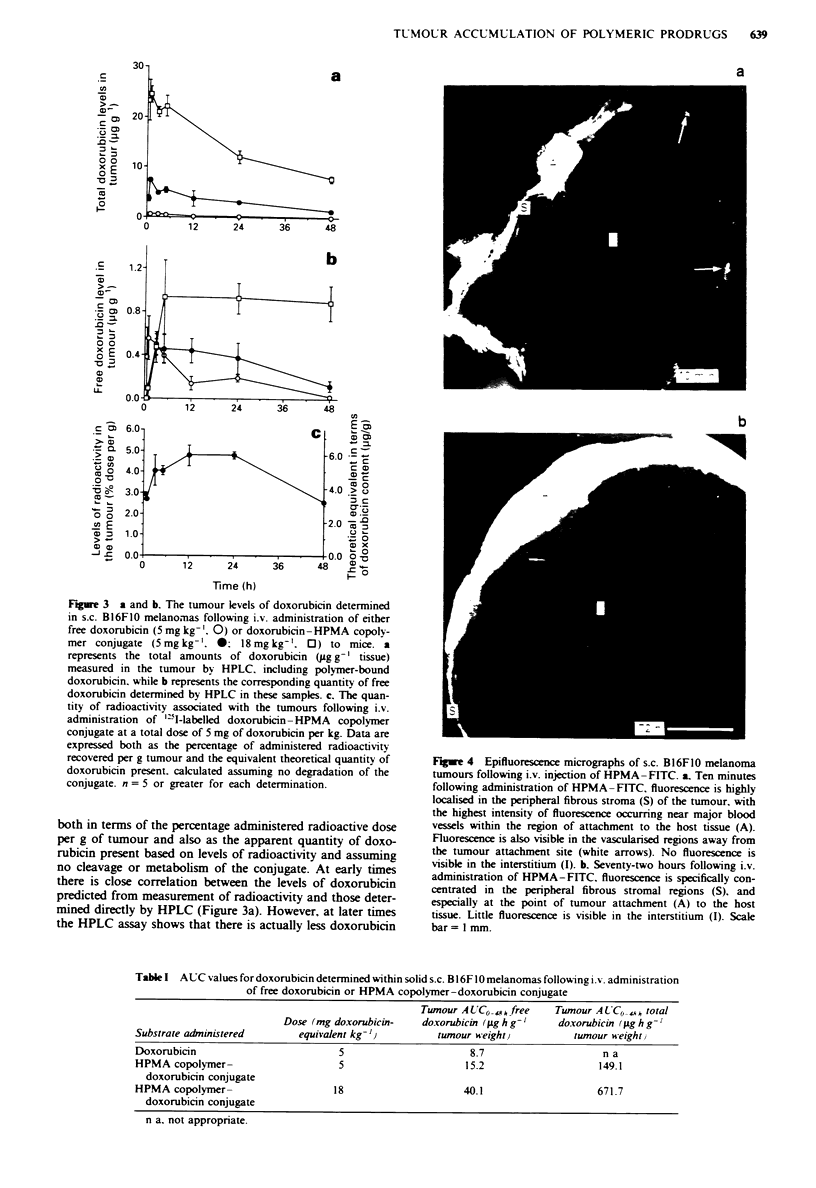
Abstract
Doxorubicin (5 mg kg-1) was administered intravenously to C57 mice bearing subcutaneous B16F10 melanomas, distributing into the tumour with an area under the concentration-time curve (0-48 h; AUC) of 8.7 micrograms h g-1. Injection of doxorubicin-N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer conjugate, containing 5 mg of doxorubicin equivalent per kg, mediated an AUC for free doxorubicin (i.e. doxorubicin released from the conjugate) of 15.2 micrograms h g-1 and for total doxorubicin (i.e. free plus conjugated) of 149.1 micrograms h g-1. An increased dose of doxorubicin-HPMA copolymer conjugate (18 mg of doxorubicin equivalent per kg) produced AUC values of 40.1 micrograms h g-1 and 671.7 micrograms h g-1 for free and total doxorubicin respectively. Hence administration of doxorubicin-HPMA copolymer conjugate achieved rises of 1.7- to 4.6-fold in tumour AUC (free doxorubicin) and 17.19 to 77.0-fold in tumour AUC (total doxorubicin). HPMA copolymers bearing fluorescein isothiocyanate accumulated in vascularised stromal regions, particularly in new growth sites at the tumour periphery. Treatment of mice with doxorubicin-HPMA copolymer conjugate achieved treated/control lifespans up to 320% (three doses of 27 mg of doxorubicin equivalent per kg) compared with only 133% using aggressive regimens of free doxorubicin (3 x 5 mg kg-1).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler T. P., Grantham F. H., Gullino P. M. Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res. 1975 Nov;35(11 Pt 1):3084–3088. [PubMed] [Google Scholar]
- Cassidy J., Duncan R., Morrison G. J., Strohalm J., Plocova D., Kopecek J., Kaye S. B. Activity of N-(2-hydroxypropyl)methacrylamide copolymers containing daunomycin against a rat tumour model. Biochem Pharmacol. 1989 Mar 15;38(6):875–879. doi: 10.1016/0006-2952(89)90274-8. [DOI] [PubMed] [Google Scholar]
- Duncan R. Drug-polymer conjugates: potential for improved chemotherapy. Anticancer Drugs. 1992 Jun;3(3):175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- Duncan R., Kopecková P., Strohalm J., Hume I. C., Lloyd J. B., Kopecek J. Anticancer agents coupled to N-(2-hydroxypropyl)methacrylamide copolymers. II. Evaluation of daunomycin conjugates in vivo against L1210 leukaemia. Br J Cancer. 1988 Feb;57(2):147–156. doi: 10.1038/bjc.1988.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., Leung D. W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990 Nov;9(3):253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- Johnston G., Benua R. S., Teates C. D., Edwards C. L., Kniseley R. M. 67Ga-citrate imaging in untreated Hodgkin's disease: preliminary report of Cooperative Group. J Nucl Med. 1974 Jun;15(6):399–403. [PubMed] [Google Scholar]
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986 Dec;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- Nelson B., Hayes R. L., Edwards C. L., Kniseley R. M., Andrews G. A. Distribution of gallium in human tissues after intravenous administration. J Nucl Med. 1972 Jan;13(1):92–100. [PubMed] [Google Scholar]
- Rihova B., Bilej M., Vetvicka V., Ulbrich K., Strohalm J., Kopecek J., Duncan R. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989 Jul;10(5):335–342. doi: 10.1016/0142-9612(89)90075-6. [DOI] [PubMed] [Google Scholar]
- Seymour L. W., Flanagan P. A., al-Shamkhani A., Subr V., Ulbrich K., Cassidy J., Duncan R. Synthetic polymers conjugated to monoclonal antibodies: vehicles for tumour-targeted drug delivery. Sel Cancer Ther. 1991 Summer;7(2):59–73. doi: 10.1089/sct.1991.7.59. [DOI] [PubMed] [Google Scholar]
- Seymour L. W. Passive tumor targeting of soluble macromolecules and drug conjugates. Crit Rev Ther Drug Carrier Syst. 1992;9(2):135–187. [PubMed] [Google Scholar]
- Seymour L. W., Ulbrich K., Strohalm J., Kopecek J., Duncan R. The pharmacokinetics of polymer-bound adriamycin. Biochem Pharmacol. 1990 Mar 15;39(6):1125–1131. doi: 10.1016/0006-2952(90)90293-t. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Honjo I., Hamamoto K., Kousaka T., Torizuka K. Positive scintiphotography of cancer of the liver with Ga 67 citrate. Am J Roentgenol Radium Ther Nucl Med. 1971 Sep;113(1):92–103. doi: 10.2214/ajr.113.1.92. [DOI] [PubMed] [Google Scholar]
- Yeung T. K., Hopewell J. W., Simmonds R. H., Seymour L. W., Duncan R., Bellini O., Grandi M., Spreafico F., Strohalm J., Ulbrich K. Reduced cardiotoxicity of doxorubicin given in the form of N-(2-hydroxypropyl)methacrylamide conjugates: and experimental study in the rat. Cancer Chemother Pharmacol. 1991;29(2):105–111. doi: 10.1007/BF00687318. [DOI] [PubMed] [Google Scholar]