Abstract
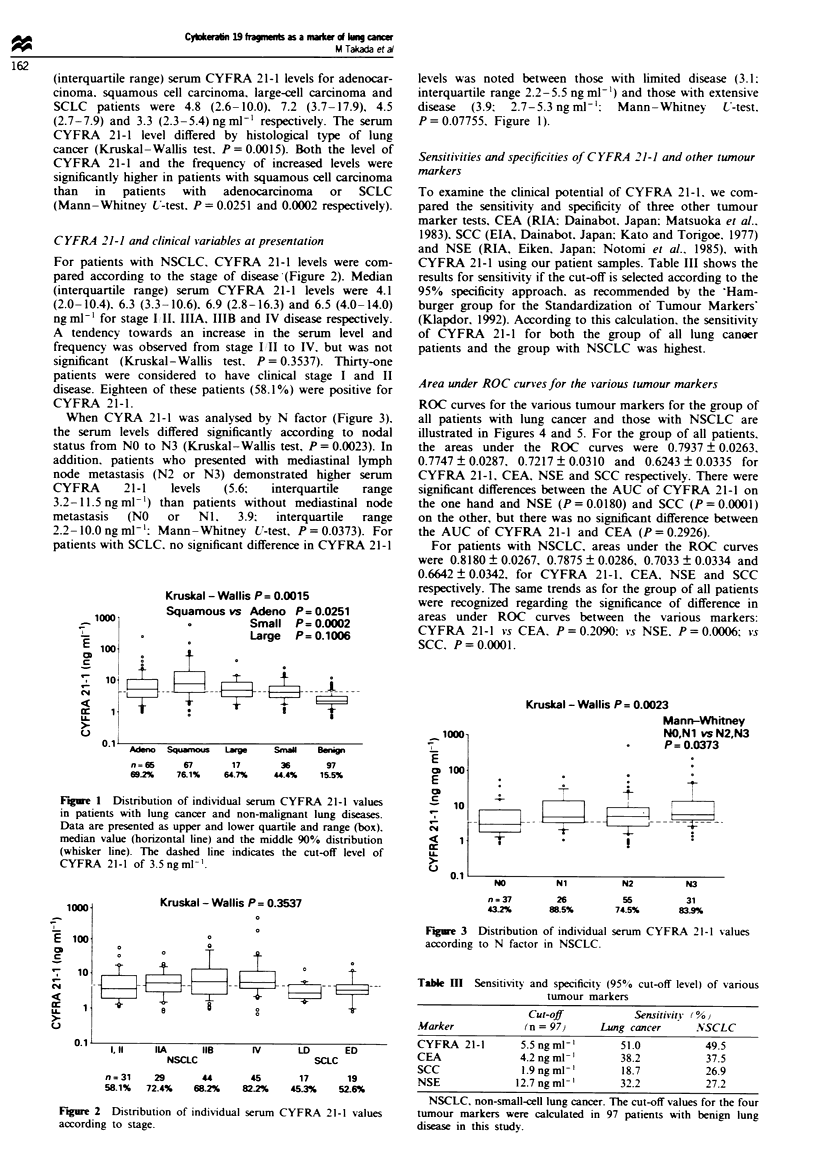
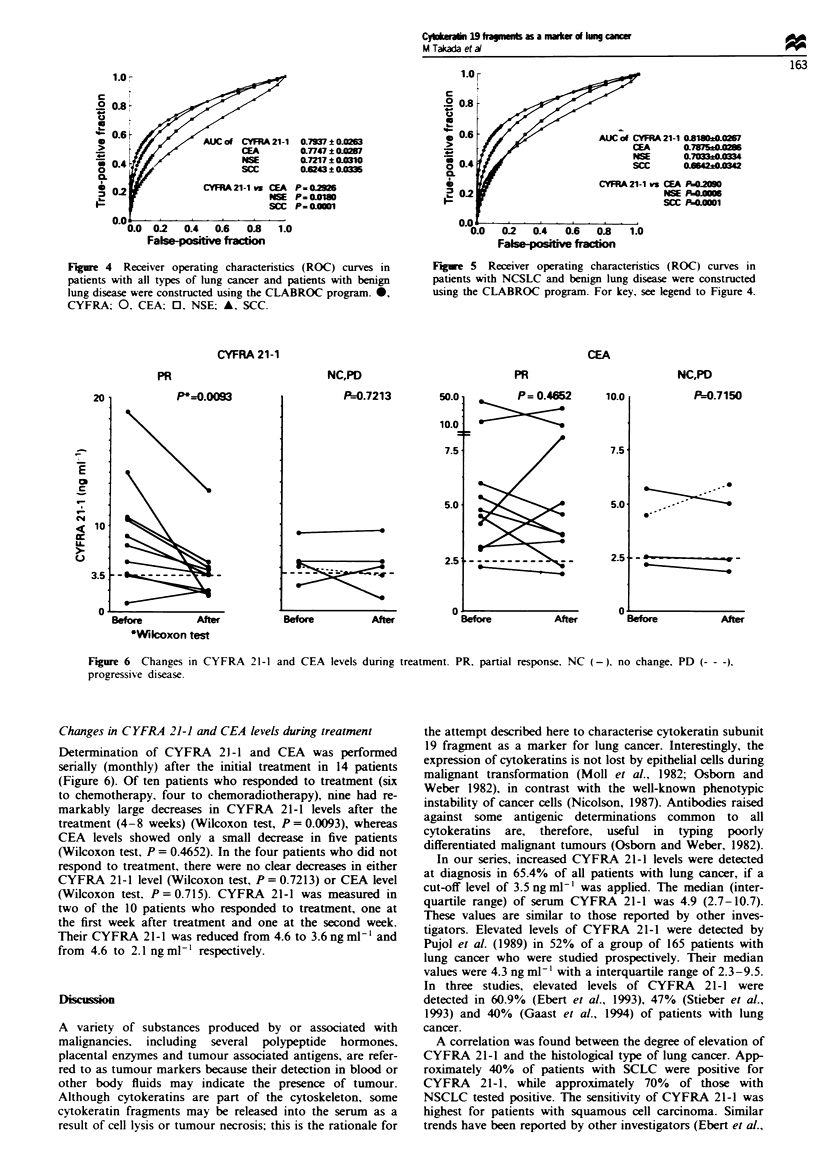
Soluble cytokeratin fragment 19 levels were measured with an enzyme immunoassay method developed by Boehringer Mannheim (Enzymun-Test CYFRA 21-1) in the serum of 185 patients with lung cancer [149 with non-small-cell lung cancer (NSCLC) and 36 with small-cell lung cancer (SCLC)] and 97 patients with benign lung diseases in order to determine its clinical usefulness in the diagnosis of lung cancer and follow-up of treatment. We used the cut-off value of 3.5 ng ml-1, established by the Japan CYFRA research group. This cut-off value is based on calculations using the receiver operating characteristic approach instead of using the 95% specificity approach recommended by other authors. The resulting sensitivity and specificity for the group of all lung cancer patients were 65.4% and 84.5% respectively. The sensitivity was highest (76.1%) for squamous cell carcinoma and lowest (44.4%) for SCLC. For NSCLC patients, when CYFRA 21-1 levels were analysed by node (N) factor, patients who presented with mediastinal lymph node metastasis (N2 or N3) demonstrated higher serum CYFRA 21-1 levels (5.6; interquartile range 3.2-11.5 ng ml-1) than patients without mediastinal node metastasis (N0 or N1, 3.9; interquartile range 2.2-10.0 ng ml-1; Mann-Whitney U-test, P = 0.0373). We compared the discriminatory power of CYFRA 21-1 with that of other tumour markers including carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC) and neuron-specific enolase (NSE). The area under the curve (AUC) of each ROC curve was calculated using the CLABROC program for statistical analysis. CYFRA 21-1 appeared to have the most discriminatory power of the markers tested in the diagnosis of lung cancer. In serial measurements of 14 patients receiving chemotherapy or radiotherapy, a high degree of correlation was noted between serum levels of CYFRA 21-1 and extent of clinical response (Wilcoxon, P = 0.0093).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akoun G. M., Scarna H. M., Milleron B. J., Bénichou M. P., Herman D. P. Serum neuron-specific enolase. A marker for disease extent and response to therapy for small-cell lung cancer. Chest. 1985 Jan;87(1):39–43. doi: 10.1378/chest.87.1.39. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Ramaekers F. C., Rot M. K., Oostendorp T., Huysmans A., van Muijen G. N., Wagenaar S. S., Vooijs G. P. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 1988 Jun 1;48(11):3221–3229. [PubMed] [Google Scholar]
- Broers J. L., Rot M. K., Oostendorp T., Huysmans A., Wagenaar S. S., Wiersma-van Tilburg A. J., Vooijs G. P., Ramaekers F. C. Immunocytochemical detection of human lung cancer heterogeneity using antibodies to epithelial, neuronal, and neuroendocrine antigens. Cancer Res. 1987 Jun 15;47(12):3225–3234. [PubMed] [Google Scholar]
- Carney D. N., Marangos P. J., Ihde D. C., Bunn P. A., Jr, Cohen M. H., Minna J. D., Gazdar A. F. Serum neuron-specific enolase: a marker for disease extent and response to therapy of small-cell lung cancer. Lancet. 1982 Mar 13;1(8272):583–585. doi: 10.1016/s0140-6736(82)91748-2. [DOI] [PubMed] [Google Scholar]
- Hansen M., Hammer M., Hummer L. Diagnostic and therapeutic implications of ectopic hormone production in small cell carcinoma of the lung. Thorax. 1980 Feb;35(2):101–106. doi: 10.1136/thx.35.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfler H., Denk H. Immunocytochemical demonstration of cytokeratin in gastrointestinal carcinoids and their probable precursor cells. Virchows Arch A Pathol Anat Histopathol. 1984;403(3):235–240. doi: 10.1007/BF00694899. [DOI] [PubMed] [Google Scholar]
- Kato H., Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977 Oct;40(4):1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Kuroki M., Koga Y., Ogawa H., Nakazawa N., Tachibana S., Minamizawa T. A new direct solid-phase radioimmunoassay for carcinoembryonic antigen without pretreatment of serum samples. J Immunol Methods. 1983 Mar 11;58(1-2):31–47. doi: 10.1016/0022-1759(83)90261-2. [DOI] [PubMed] [Google Scholar]
- McKenzie C. G., Evans I. M., Hillyard C. J., Hill P., Carter S., Tan M. K., MacIntyre I. Biochemical markers in bronchial carcinoma. Br J Cancer. 1977 Dec;36(6):700–707. doi: 10.1038/bjc.1977.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Mountain C. F. A new international staging system for lung cancer. Chest. 1986 Apr;89(4 Suppl):225S–233S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Notomi T., Morikawa J., Kato K., Tsuchida Y., Ohsawa R. Radioimmunoassay development for human neuron-specific enolase: with some clinical results in lung cancers and neuroblastoma. Tumour Biol. 1985;6(1):57–66. [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Pujol J. L., Grenier J., Daurès J. P., Daver A., Pujol H., Michel F. B. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993 Jan 1;53(1):61–66. [PubMed] [Google Scholar]
- Pujol J. L., Simony J., Laurent J. C., Richer G., Mary H., Bousquet J., Godard P., Michel F. B. Phenotypic heterogeneity studied by immunohistochemistry and aneuploidy in non-small cell lung cancers. Cancer Res. 1989 May 15;49(10):2797–2802. [PubMed] [Google Scholar]
- Stieber P., Hasholzner U., Bodenmüller H., Nagel D., Sunder-Plassmann L., Dienemann H., Meier W., Fateh-Moghadam A. CYFRA 21-1. A new marker in lung cancer. Cancer. 1993 Aug 1;72(3):707–713. doi: 10.1002/1097-0142(19930801)72:3<707::aid-cncr2820720313>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Waalkes T. P., Abeloff M. D., Woo K. B., Ettinger D. S., Ruddon R. W., Aldenderfer P. Carcinoembryonic antigen for monitoring patients with small cell carcinoma of the lung during treatment. Cancer Res. 1980 Dec;40(12):4420–4427. [PubMed] [Google Scholar]
- van der Gaast A., Schoenmakers C. H., Kok T. C., Blijenberg B. G., Cornillie F., Splinter T. A. Evaluation of a new tumour marker in patients with non-small-cell lung cancer: Cyfra 21.1. Br J Cancer. 1994 Mar;69(3):525–528. doi: 10.1038/bjc.1994.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


