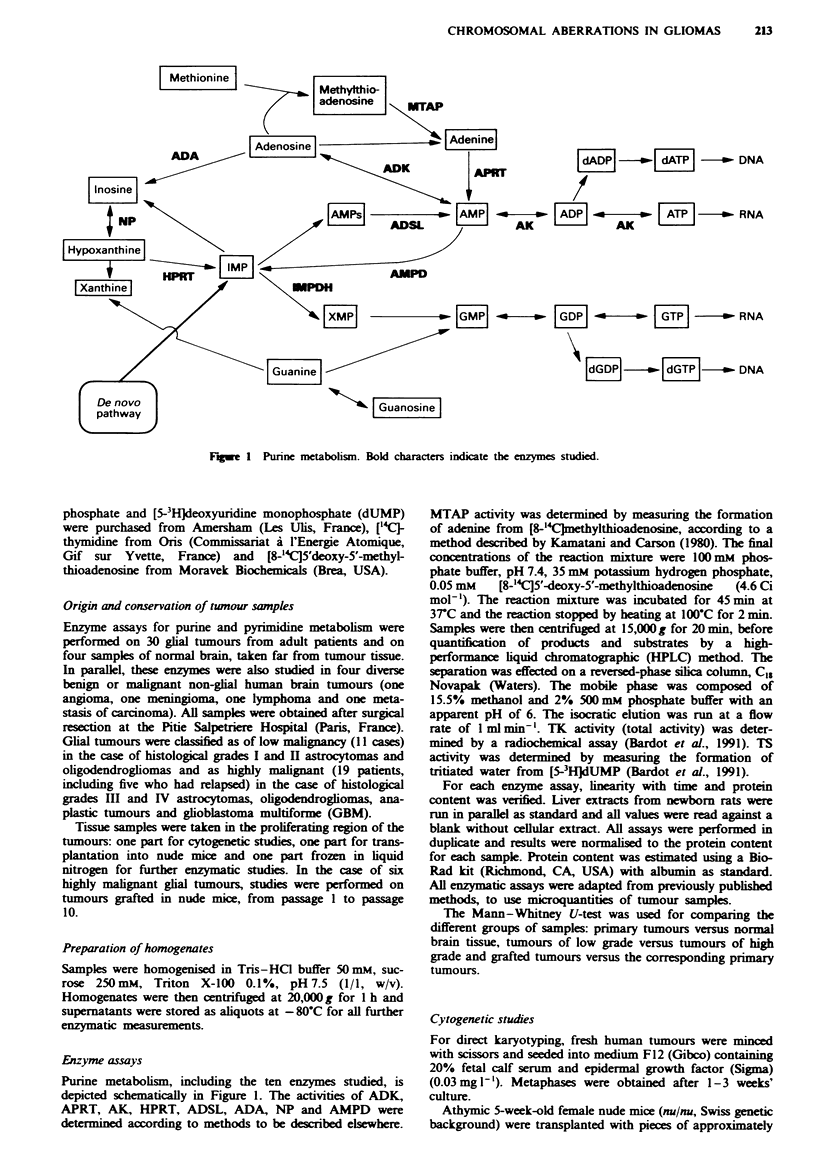
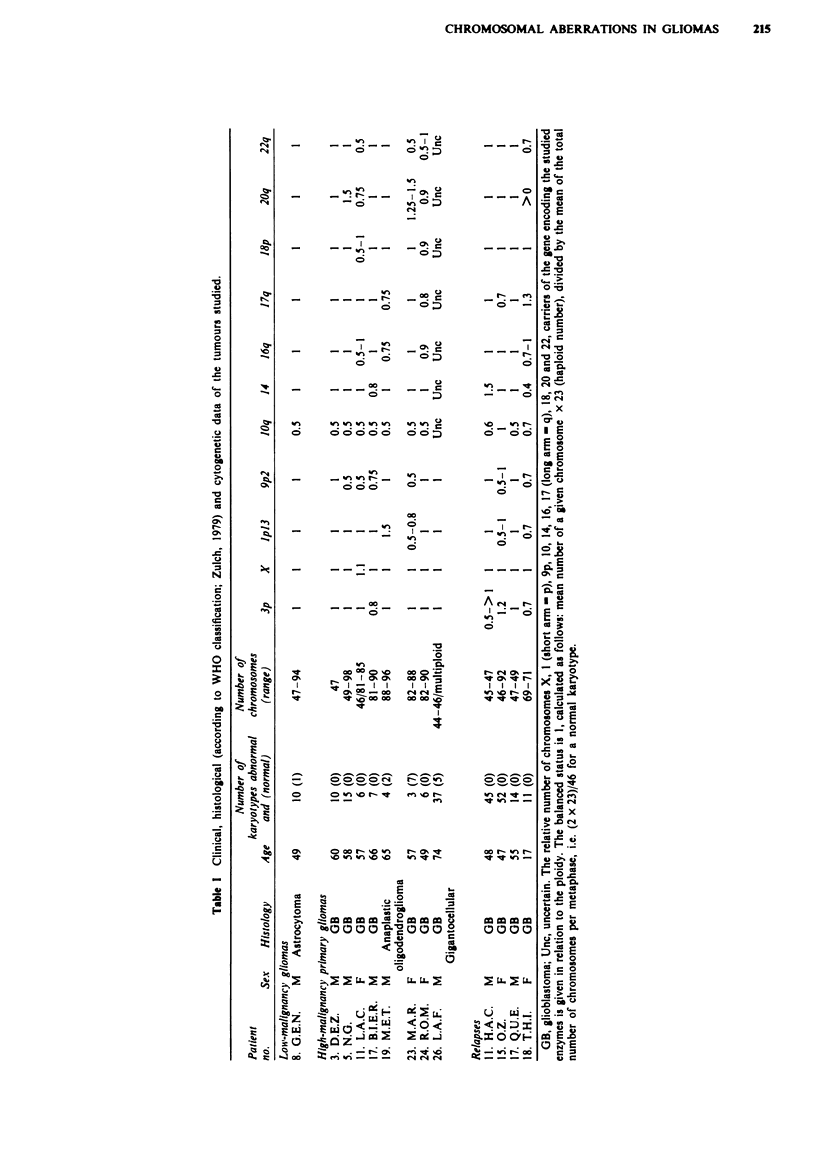
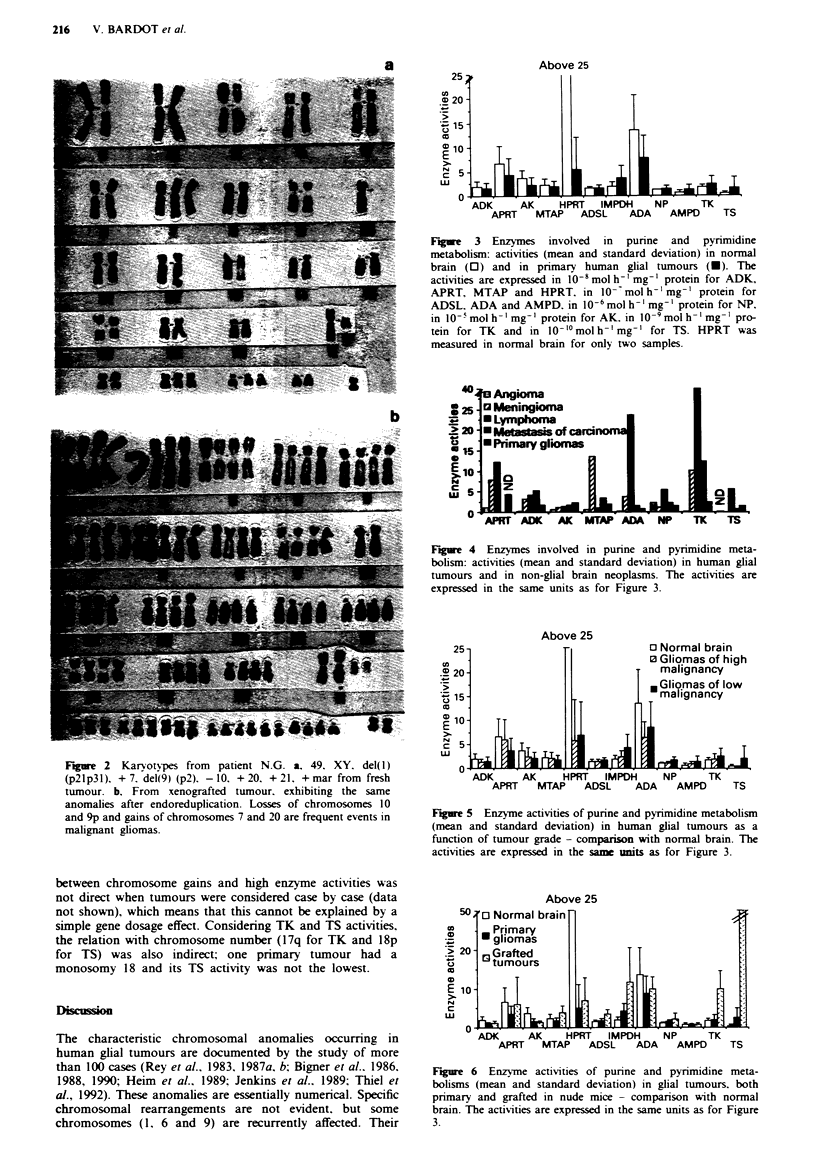
Abstract
Chromosomal aberrations in human gliomas are principally numerical. In tumours of low malignancy, karyotypes are frequently normal, but occasionally an excess of chromosome 7 and a loss of sex chromosome are observed. In highly malignant tumours, the most frequent aberrations are gain of chromosome 7, loss of chromosome 10 and less frequently losses or deletions of chromosomes 9, 22, 6, 13 and 14 or gains of chromosomes 19 and 20. To understand the meaning of these chromosome imbalances, the relationships between chromosome abnormalities and metabolic disturbances were studied. The losses or deletions observed affected principally chromosomes carrying genes encoding enzymes involved in purine metabolism. The activities of ten enzymes were measured: adenosine kinase, adenine phosphoribosyltransferase, adenylate kinase, methylthioadenosine phosphorylase, hypoxanthine phosphoribosyltransferase, adenylosuccinate lyase, inosine monophosphate dehydrogenase, adenosine deaminase, nucleoside phosphorylase and adenosine monophosphate deaminase. In parallel, two enzymes involved in pyrimidine metabolism, thymidine kinase and thymidylate synthase (TS), were studied. The activities of all these enzymes were measured on samples from 30 human primary glial tumours with low or high malignancy, six xenografted tumours at different passages, four portions of normal brain tissue and four non-glial brain neoplasms. As suggested by cytogenetic data, the enzymatic results showed a relatively low activity of purine metabolism in glial tumours when compared with normal brain and non-glial brain neoplasms. Considering the two enzymes involved in pyrimidine metabolism, only TS had higher activity in glial tumours of high malignancy than in normal brain. In comparison with normal brain, the balance between salvage and de novo pathways changes in gliomas, and even more in grafted tumours, in favour of de novo synthesis. The relation between chromosomes and metabolic imbalances does not correspond to a simple gene dosage effect in these tumours. These data suggest that the decrease of adenosine metabolism occurs before chromosomal aberrations appear, since it is observed in tumours of low malignancy when most karyotypes are still normal, and that the de novo pathway increases with tumour progression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardot V., Luccioni C., Lefrançois D., Muleris M., Dutrillaux B. Activity of thymidylate synthetase, thymidine kinase and galactokinase in primary and xenografted human colorectal cancers in relation to their chromosomal patterns. Int J Cancer. 1991 Mar 12;47(5):670–674. doi: 10.1002/ijc.2910470507. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bigner D. D. Cytogenetics of human brain tumors. Cancer Genet Cytogenet. 1990 Jul 15;47(2):141–154. doi: 10.1016/0165-4608(90)90024-5. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bullard D. E., Mahaley M. S., Jr, Bigner D. D. Chromosomal evolution in malignant human gliomas starts with specific and usually numerical deviations. Cancer Genet Cytogenet. 1986 Jun;22(2):121–135. doi: 10.1016/0165-4608(86)90172-x. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Bravard A., Luccioni C., Muleris M., Lefrancois D., Dutrillaux B. Relationships between UMPK and PGD activities and deletions of chromosome 1p in colorectal cancers. Cancer Genet Cytogenet. 1991 Oct 1;56(1):45–56. doi: 10.1016/0165-4608(91)90361-w. [DOI] [PubMed] [Google Scholar]
- Couturier J., Vielh P., Salmon R. J., Lombard M., Dutrillaux B. Chromosome imbalance in endometrial adenocarcinoma. Cancer Genet Cytogenet. 1988 Jul 1;33(1):67–76. doi: 10.1016/0165-4608(88)90051-9. [DOI] [PubMed] [Google Scholar]
- Dutrillaux B., Muleris M. Induction of increased salvage pathways of nucleotide synthesis by dosage effect due to chromosome imbalances may be fundamental in carcinogenesis: the example of colorectal carcinoma. Ann Genet. 1986;29(1):11–15. [PubMed] [Google Scholar]
- Heim S., Mandahl N., Jin Y., Strömblad S., Lindström E., Salford L. G., Mitelman F. Trisomy 7 and sex chromosome loss in human brain tissue. Cytogenet Cell Genet. 1989;52(3-4):136–138. doi: 10.1159/000132863. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Weber G., Morris H. P. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975 Jul 24;256(5515):331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Jenkins R. B., Kimmel D. W., Moertel C. A., Schultz C. G., Scheithauer B. W., Kelly P. J., Dewald G. W. A cytogenetic study of 53 human gliomas. Cancer Genet Cytogenet. 1989 Jun;39(2):253–279. doi: 10.1016/0165-4608(89)90192-1. [DOI] [PubMed] [Google Scholar]
- Kamatani N., Carson D. A. Abnormal regulation of methylthioadenosine and polyamine metabolism in methylthioadenosine phosphorylase-deficient human leukemic cell lines. Cancer Res. 1980 Nov;40(11):4178–4182. [PubMed] [Google Scholar]
- Lefrançois D., Olschwang S., Delattre O., Muleris M., Dutrillaux A. M., Thomas G., Dutrillaux B. Preservation of chromosome and DNA characteristics of human colorectal adenocarcinomas after passage in nude mice. Int J Cancer. 1989 Nov 15;44(5):871–878. doi: 10.1002/ijc.2910440521. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Berger S. J., Carter J. G., Chi M. M., Manchester J. K., Knor J., Pusateri M. E. Diversity of metabolic patterns in human brain tumors: enzymes of energy metabolism and related metabolites and cofactors. J Neurochem. 1983 Oct;41(4):994–1010. doi: 10.1111/j.1471-4159.1983.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Berger S. J., Chi M. M., Carter J. G., Blackshaw A., Outlaw W. Diversity of metabolic patterns in human brain tumors--I. High energy phosphate compounds and basic composition. J Neurochem. 1977 Dec;29(6):959–977. doi: 10.1111/j.1471-4159.1977.tb06500.x. [DOI] [PubMed] [Google Scholar]
- Luccioni C., Muleris M., Sabatier L., Dutrillaux B. Chromosomal and enzymatic patterns provide evidence for two types of human colon cancers with abnormal nucleotide metabolism. Mutat Res. 1988 Jul-Aug;200(1-2):55–62. doi: 10.1016/0027-5107(88)90071-1. [DOI] [PubMed] [Google Scholar]
- Mangiardi J. R., Yodice P. Metabolism of the malignant astrocytoma. Neurosurgery. 1990 Jan;26(1):1–19. doi: 10.1097/00006123-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Marzatico F., Curti D., Dagani F., Silvani V., Gaetani P., Butti G., Knerich R. Enzymes related to energy metabolism in human gliomas. J Neurosurg Sci. 1986 Jul-Sep;30(3):129–132. [PubMed] [Google Scholar]
- Natsumeda Y., Prajda N., Donohue J. P., Glover J. L., Weber G. Enzymic capacities of purine de Novo and salvage pathways for nucleotide synthesis in normal and neoplastic tissues. Cancer Res. 1984 Jun;44(6):2475–2479. [PubMed] [Google Scholar]
- Paulus W., Peiffer J. Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer. 1989 Jul 15;64(2):442–447. doi: 10.1002/1097-0142(19890715)64:2<442::aid-cncr2820640217>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Pillwein K., Chiba P., Knoflach A., Czermak B., Schuchter K., Gersdorf E., Ausserer B., Murr C., Goebl R., Stockhammer G. Purine metabolism of human glioblastoma in vivo. Cancer Res. 1990 Mar 1;50(5):1576–1579. [PubMed] [Google Scholar]
- Rey J. A., Bello M. J., de Campos J. M., Benítez J., Ayuso M. C., Valcárcel E. Chromosome studies in two human brain tumors. Cancer Genet Cytogenet. 1983 Oct;10(2):159–165. doi: 10.1016/0165-4608(83)90120-6. [DOI] [PubMed] [Google Scholar]
- Rey J. A., Bello M. J., de Campos J. M., Kusak M. E., Moreno S. Chromosomal composition of a series of 22 human low-grade gliomas. Cancer Genet Cytogenet. 1987 Dec;29(2):223–237. doi: 10.1016/0165-4608(87)90233-0. [DOI] [PubMed] [Google Scholar]
- Rey J. A., Bello M. J., de Campos J. M., Kusak M. E., Ramos C., Benitez J. Chromosomal patterns in human malignant astrocytomas. Cancer Genet Cytogenet. 1987 Dec;29(2):201–221. doi: 10.1016/0165-4608(87)90232-9. [DOI] [PubMed] [Google Scholar]
- Shapiro J. R. Biology of gliomas: heterogeneity, oncogenes, growth factors. Semin Oncol. 1986 Mar;13(1):4–15. [PubMed] [Google Scholar]
- Thiel G., Losanowa T., Kintzel D., Nisch G., Martin H., Vorpahl K., Witkowski R. Karyotypes in 90 human gliomas. Cancer Genet Cytogenet. 1992 Feb;58(2):109–120. doi: 10.1016/0165-4608(92)90095-p. [DOI] [PubMed] [Google Scholar]
- Venter D. J., Thomas D. G. Multiple sequential molecular abnormalities in the evolution of human gliomas. Br J Cancer. 1991 May;63(5):753–757. doi: 10.1038/bjc.1991.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung W. K., Lotan R., Lee P., Lotan D., Steck P. A. Modulation of growth and epidermal growth factor receptor activity by retinoic acid in human glioma cells. Cancer Res. 1989 Feb 15;49(4):1014–1019. [PubMed] [Google Scholar]