Abstract
In the progeny of the monosomic addition line of common wheat, Triticum aestivum, carrying the gametocidal chromosome of Aegilops cylindrica, deletion chromosomes carrying the break point within the nucleolar organizing region of chromosome 1B appeared. Attempts were made to amplify the break points by PCR using primers of telomere and rDNA (rRNA gene). In one deletion line, specific amplification of DNA fragments including the 18S rRNA gene, telomere repeats, and their junction occurred. At the junction of telomere and rRNA gene there was a 31-bp inverted duplication of the rRNA gene. Telomere sequences were initiated from the sequence TAG in the duplication. Between the duplications a small sequence was also inserted. This novel DNA sequence at the break point indicates that the breakage–fusion–bridge cycle(s) took place after the first chromatin breakage by the gametocidal gene.
Keywords: chromosome breakage, telomere, telomerase, Triticum aestivum
Detailed observation of broken maize chromosome by McClintock (1) showed that broken chromosome ends were sticky, a condition that subsequently produced new chromosome aberrations through breakage–fusion–bridge (BFB) cycles. The BFB cycles took place only in specific developmental stages such as gametogenesis and endosperm formation, and not in embryogenesis, during which the broken ends healed and did not begin BFB cycles. Broken chromosome ends in other organisms showed behaviors similar to those in maize. The healing is now explained by formation of telomere repetitive sequences at the broken ends by the action of telomerase. In the case of human broken chromosomes concerned with α thalassemia, the healed broken ends, located at a very distal region of chromosome 16, were found to carry the telomere repetitive sequences (2).
Tsujimoto and Tsunewaki (3, 4) reported that alien chromosomes carrying gametocidal (Gc) genes caused chromosome breakage at a high frequency in the genetic background of common wheat, Triticum aestivum. The Gc genes, in the hetero- and hemizygous condition, induce chromosome breakage in the gametes not carrying the gene. In addition, some Gc genes cause chromosome breakage during early embryogenesis when the male gametes with the gene fertilize the female gametes lacking it (5).
The molecular mechanism of the chromosome breakage by Gc genes is not understood. However, Gc genes have been widely used to produce hundreds of chromosome deletion lines in wheat (6, 7). The deletion lines are very useful for mapping genes and molecular markers on wheat chromosomes (8, 9, 10, 11). Cytologically, many deletion lines seem to be stable from generation to generation. In addition, in situ hybridization with the probe of telomeric sequences showed signals at broken ends of the deletion chromosomes of wheat (12, 13). The telomere signal intensity increased gradually during development (13), suggesting that the broken ends were healed after the breakage. Wheat plants with chromosome deletions that are stably transmitted to the next generation will be suitable materials for studies to reveal the nature of telomerase to seed the telomere repeat at the broken ends.
Many chromosomes with deletions at the nucleolar organizing region (NOR) of chromosome 1B were produced (ref. 6; unpublished work). This region consists of thousands of tandem repeats of 18S and 26S rRNA gene (rDNA) units (9 kb per unit; ref. 14). If telomere repetitive sequences are present at the break point of NOR, then the DNA fragments including the break point can be amplified by PCR using primers designed from the rDNA and telomere sequences.
In this paper we show the DNA sequence of a break point that suggests the occurrence of a BFB event(s) after chromosome breakage by the Gc gene.
MATERIALS AND METHODS
Plant Materials.
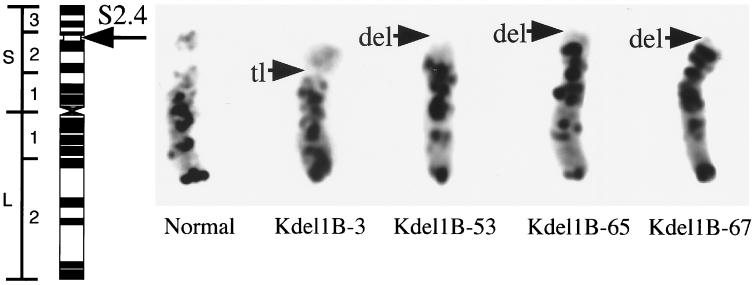
Common wheat (Triticum aestivum L. em. Thell.; 2n = 6x = 42), cultivar Chinese Spring (CS), and eight lines carrying chromosome aberrations at the NOR (S2.4 region) of chromosome 1B were used in this study (Fig. 1). These chromosome lines were selected by us (unpublished work) and Endo and Gill (6) among the progeny of the monosomic addition line bearing the chromosome 2C of Aegilops cylindrica Host (2n = 43 = 21" + 1′). The gametocidal gene(s) on this alien chromosome induces chromosome breakage in the gametes without the gene (16).
Figure 1.
Wheat chromosome 1B with a deletion or a translocation used in this study. Only the chromosomes selected in our laboratory are shown. On the left an idiogram of the chromosome 1B with the regional designation (15) is shown. All chromosomes cytologically seem to have the break point at the NOR of the short arm (indicated by arrows).
Chromosome 1B is one of the sat-chromosomes. Region S2.4 of this chromosome is located in the NOR, thus in the repeats of 18S-26S rDNA and secondary constriction (Fig. 1; see ref. 15 for banding nomenclature). In two lines, Kdel1B-53 and Kdel1B-65, chromosome 1B lacked the distal region of S2.4; thus, they were deemed cytologically to have lost the satellite. Line Kdel1B-67 was selected as a carrier of chromosome 1B without the satellite in an earlier generation (Fig. 1), but later this was found to carry a larger deletion, the break point of which was located at S1.6. The satellite of chromosome 1B in the line Kdel1B-3 is larger than that of the normal chromosome 1B, a fact that may be due to a translocation within the S2.4 region. The four lines of Endo and Gill (6) used in this study (lines 1BS-2, 1BS-5, 1BS-8, and 1BS-16) lacked the satellite of the chromosome 1B (17).
For C-banding, the technique described by Tsujimoto and Niwa (18) was used.
PCR Analyses.
The genomic DNAs were isolated from the leaves by following the procedure of Appels and Moran (19) as modified by Koebner et al. (20). PCR was carried out in 50 μl of reaction solution mixture [10 mM Tris·HCl (pH 8.3); 50 mM KCl; 1.5 mM MgCl2; 0.2 mM each of dATP, dGTP, dCTP, and dTTP; and 1.25 units of Taq DNA polymerase (Takara Shuzo, Kyoto)] with the templates of the plant DNAs (0.2 μg) and each combination of primers of rDNA and telomere (0.25 μM each). The primers of the rDNA and telomere were designed on the basis of the sequences of ribosomal 18S-26S RNA gene repeats of wheat or tomato (Fig. 2) and telomere sequences of Arabidopsis thaliana (ref. 24, Table 1). Temperature conditions for the PCR were 95°C for 2 min followed by 32 cycles of 94°C for 1 min, 64°C for 2 min, and 72°C for 3 min. These conditions amplify the expected DNA fragments of unique genomic sequences (25).
Figure 2.
Positions of PCR primers on a unit of 18S.26S rDNA of wheat. Boxes indicate 26S and 18S rRNA genes, and the line is their intergenic spacer region. In hatched boxes and line, primers were designed following the wheat sequence (21); and in vacant boxes, following the tomato sequence (22, 23). “RDN” of the primer names is omitted here (see Table 1).
Table 1.
Names and sequences of PCR primers used in this study
| Name | Sequence (5′→ 3′) | Ref. |
|---|---|---|
| Telo20 | CTAAACCCTAAACCCTAAAC | Arabidopsis telomere (24) |
| RDN-1 | CGTGATTTGGCCTTGCAGGA | Wheat rDNA (21) |
| RDN-2 | CATGTGTGTGCAACTCATCA | Wheat rDNA |
| RDN-3 | ATTCAACCTAGTACGAGAGG | Tomato 25S rRNA gene (22) |
| RDN-4 | TTTGGTAAGCAGAACTGGCC | Tomato 25S rRNA gene |
| RDN-5 | TCAAGAACGAAAGTTGGGGG | Tomato 17S rRNA gene (23) |
| RDN-R1 | TGCCTTCCTTGGATGTGGTA | Wheat rDNA |
| RDN-R2 | GGGACCCCTGCCCTTAGTTT | Wheat rDNA |
| RDN-R3 | TAGTCCTCATGATTTTGCGG | Wheat rDNA |
| RDN-R4 | CGCGGCGACCCGCTCTCGCC | Tomato 25S rRNA gene |
| RDN-R5 | GCCGCTGAGGACGCTTCTCC | Tomato 25S rRNA gene |
Cloning and Sequencing.
For cloning PCR fragments, deoxythymidine was added to the ends of SmaI-digested pUC18 fragments by a 30-min incubation at 74°C in 100 μl of solution (10 mM Tris·HCl, pH 8.3/50 mM KCl/1.5 mM MgCl2/0.5 mM dTTP/2.5 units of Taq DNA polymerase). A ligation kit (Takara Shuzo) was used to link this vector with the PCR fragments. Escherichia coli strain DH5 was transformed by this DNA. Clones for sequence were chosen by the cracking-PCR method, and the plasmid DNAs were isolated by the alkaline lysis method. The sequences were determined by dideoxynucleotide terminating reaction carried out with a Sequitherm Kit (Epicentre Technologies, Madison, WI) and an autosequencer (Li-Cor, Lincoln, NE). They were analyzed by the computer software genetyx (Software Development, Tokyo) for the homology.
RESULTS
Specific Amplification of DNA Fragments.
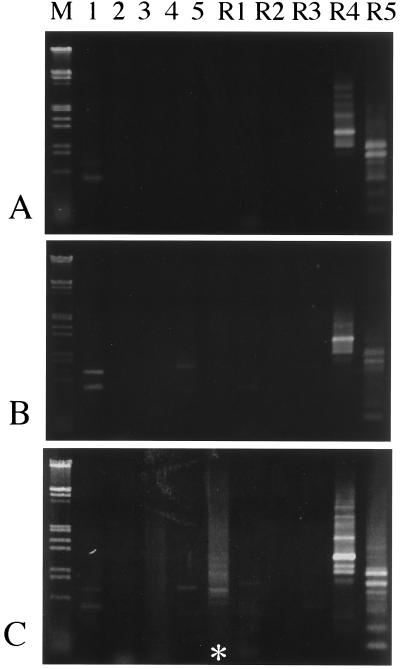
First, using normal CS DNA, the ability of the primers to amplify specific unique sequences was examined. The primers RDN-1, RDN-R4, and RDN-R5 (Table 1) amplified certain fragments by themselves, without the telomere primer, Telo20, indicating that in wheat genomes pairs of those primer sequences were located in inverse direction within the length of the DNA amplified by PCR (Fig. 3A). Moreover, since the amount and numbers of the DNA fragments amplified by the primer RDN-R4 or RDN-R5 were much greater than expected from the PCR using primers of single-copy sequences, there must be many inverted sites of RDN-R4 and RDN-R5 sequences in the genomes. PCR with other primers did not show such remarkable amplification of DNA fragments.
Figure 3.
Specific amplification of DNA fragments in the deletion line Kdel1B-65. (A) PCR with one of the rDNA primers denoted on the lanes (“RDN” of the primer names is omitted here) and the template of normal CS DNA. (B) PCR with combination of primers of telomere (Telo20) and one of the rDNA primers above, and the template of CS. (C) The same PCR as in B except the template DNA of deletion line Kdel1B-65 was used. Specific amplification that appeared in the lane containing RDN-5 is indicated by an asterisk. M is the molecular weight marker, λ phage DNA digested with EcoRI and HindIII.
With the template of normal CS DNA, the combinations of Telo20 and some of the rDNA primers amplified DNA fragments in addition to those mentioned above (Fig. 3B). Most of these fragments appeared also in deletion or translocation lines used.
The PCR with the primers RDN-5 and Telo20 on the template of Kdel1B-65 DNA amplified specific DNA fragments that did not appear in the PCR with other templates (Fig. 3C). The Kdel1B-65-specific fragments were varied in size and produced a smear pattern in the gel over an area indicating a mode of about 700 bp. These fragments must include the rDNA sequence and various lengths of the telomere repeats.
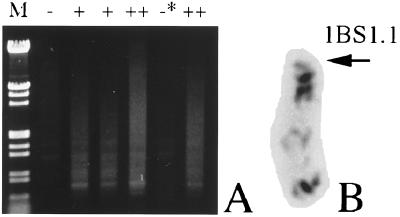
In the selfed progeny of the hemizygotes for this deletion chromosome, the specific amplification appeared only in the plants carrying the deletion chromosome (Fig. 4A). In this progeny one plant lacking the whole short arm of the chromosome 1B also appeared (Fig. 4B); it may have been produced by misdivision of the deletion chromosome. This plant also did not show the specific amplification of the fragments, indicating that the amplified sequence was located on the broken short arm of the deletion chromosome in Kdel1B-65.
Figure 4.
(A) Segregation of the specific amplification of the DNA fragments in the progeny of a heterozygote for the deletion 1B chromosome of Kdel1B-65. Homozygotes and hemizygotes are indicated by “++” and “+”, respectively, on the lanes. Plant without chromosome 1B is indicated by “−”. Plant with telocentric chromosome without the whole short arm (shown in B) is indicated by “−*”.
Sequence of the Specific DNA Fragments.
The PCR-amplified fragments were cloned, and four of the clones were sequenced (Fig. 5). All four included identical sequences, indicating that they might have originated from the same location of the chromosome. They comprised the sequences of the 18S rRNA gene, telomere repeats, and their junction.
Figure 5.
Structure and DNA sequence of a clone, pTaB1B65, including a break point of the Kdel1B-65 line. The 18S rRNA gene and telomere regions are boxed with designations. Thick arrow indicates inverted duplication at break point and junction region. Dotted arrows indicate the primers. The other remarkable sequences are shown in boldface type (see text).
The 18S rRNA region had high homology with tomato and rice 18S rRNA genes (95.5% and 97.9% identity, respectively). The length of the telomere region varied among the clones. This must be because the primer Telo20 could hybridize at any unit of the telomere repeats.
A specific sequence of 39 bp was inserted between the 18S rRNA gene and the telomere. Most of the sequence of the insertion (31 bp from its 3′ end) was inversion of the 18S rRNA gene from the border between the rRNA gene and the insertion. The remaining 8-bp sequence was unknown. The last three nucleotides of the inverted sequence were TAG, which is present in the telomere repeat unit of plants.
The telomere region following the TAG sequence mostly consisted of the repeats of TTTAGGG as found in Arabidopsis and other higher plants (24, 26, 27). Their variant, TTAGGG, which is found in the mammalian telomere, frequently appeared in the telomere repeats (Table 2). In the clone carrying the longest telomere region, the frequency of this variant was 21.8%. One novel sequence, TATATA, was inserted in the region. No TTTTGGG, which was reported as a frequent telomere variant in plants, appeared in our clones. All the clones carried the same variation at the same position, again indicating that they came from a single locus.
Table 2.
Consensus and variant telomere repetitive sequences synthesized at the breakage point of chromosome 1B of line Kdel1B-65
| Sequence | No. of repeats | Frequency, % |
|---|---|---|
| TTTAGGG | 60 | 75.9 |
| TTAGGG | 17 | 21.5 |
| TTTGGGG | 1 | 1.3 |
| TATATA | 1 | 1.3 |
DISCUSSION
Events Deduced from DNA Structure at the Chromosome Break Point.
In higher eukaryotes in which chromosome breakage is an accidental event, specific DNA structures at the broken chromosome ends have not been reported except for the megabase-sized inverted duplication as the result of chromatid-type BFB cycles (28).
Our present study on common wheat showed a novel sequence at the break point—i.e., at the junction between the 18S rRNA gene and the telomere sequences. The specific sequence consisted of 8 bp of unknown nucleotides and an inverted 31-bp sequence of which the last 3 bp could be a possible initiation site of the telomerase.
This novel sequence may be the remnant of the specific breakage event caused by the gametocidal gene. In fact, Ogihara et al. (11) and Hohmann et al. (29) reported unexpected chromosome rearrangement in the deletion lines caused by the gametocidal genes by unexpected loss of RFLP (restriction fragment length polymorphism) markers in the deletion lines.
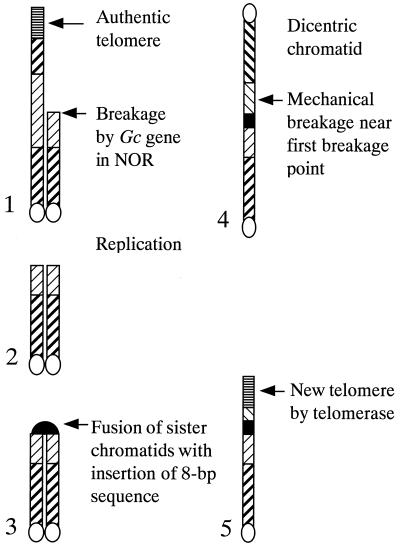
More probably, this structure may be attributed to the occurrence of the BFB cycle(s) before addition of the telomere sequences at the sticky end (Fig. 6). If a breakage in a chromatid is followed by replication and fusion, symmetrical dicentric chromosome around the break point would be the result. In the process of fusion the 8-bp DNA sequence was inserted. In the following anaphase, the dicentric chromosome was mechanically broken. In the present case the mechanical breakage must have occurred at a position near the first point of breakage caused by the Gc gene. This breakage may have exposed, at the end, the sequence TAG that can be a possible substrate of telomerase, or more possibly, some digestion by endogenous exonuclease may have opened the sequence. Finally, the endogenous telomerase synthesized the telomere repeats to the sequence. Because the first and the second breakage were close to each other, the inverted duplication was only 31 bp. McClintock (1) in maize and Lukaszewski (30) in wheat observed cytologically that in BFB cycles the fused points tended to break in the following breakage stage. BFB cycles are widely observed after chromosome breakage in many organisms. However, there has been no molecular evidence for such an event. The rearranged DNA sequence at the break point in this study can be explained by the occurrence of BFB cycles.
Figure 6.
Production of inverted duplication at the break point of chromosome 1B by the BFB cycle. Only the short arm is shown. The rDNA region and the inserted 8-bp sequences are indicated by hatched and thick boxes, respectively.
It is already known that the chromosome breakage by the Gc gene of Aegilops cylindrica occurs in cells of gametophytes (31). In the monosomic addition lines with the chromosome carrying Gc gene(s), the genotypes of the egg cell and polar nuclei of a single embryo sac, both of which originate from a single megaspore, are not always the same (unpublished results). Once the chromosomes are broken by the Gc gene, the broken ends gradually acquire the telomere sequences and heal (13). The present results indicate that before the healing one BFB cycle took place in the line Kdel1B-65.
Direction of rDNA Repeats on the Chromosome.
In the chromosome deletion line Kdel1B-65, the chromosome breakage was found to have occurred within an 18S rRNA gene of the rDNA repeats. The upstream sequence of the 18S rRNA gene was maintained and followed to the specific junction structure and telomere sequences. This finding indicates that the upstream region of the genes is located toward the proximal direction of the chromosome at this site; i.e., the rRNAs were transcribed in the proximal to the distal direction on the chromosome.
Telomere Sequences.
In plants, telomere sequences were first isolated from a normal chromosome end of Arabidopsis thaliana (24) and later from normal chromosome ends of maize and barley (26, 27). All the sequences consisted of 7-bp repeats, TTTAGGG, with some variations on the basic 7-bp motif. The present wheat telomere sequence at the broken end also consisted of TTTAGGG with variants such as TTAGGG, TTTGGGG, and TATATA (Table 2). These variants are not frequent in plant telomeres reported, though the first variant is the telomere sequence of mammals.
There are 2- to 4-kb (Arabidopsis) to more than 25-kb (barley) arrays of telomere repeats in plants (24, 32). Most of the proximal sequences in the arrays are not plausibly synthesized by telomerase but by ordinary DNA polymerase in semiconservative replication. Previously cloned telomere sequences in plants did not always come from the real termini of the telomere sequences directly synthesized by telomerase. These may have been maintained by semiconservative replication for a long time and may have become uniform by recombination, gene conversion, unequal crossing-over, or other mechanisms as seen in the case of other repetitive sequences. On the other hand, the present sequences at the broken end are newly synthesized ones after breakage by the Gc gene. The variants in the present sequence may be attributable to the variation of RNA sequences in wheat telomerase or to the ability of this enzyme to transcribe DNA accurately. Alternatively, “SOS telomere synthesis” at the accidentally broken chromosome ends may not be the same as that at authentic telomeres reported previously.
Previous reports on broken human chromosome ends showed the sequence GTT or GGGT as the telomere initiation site (2, 33). In this study the telomere sequences were initiated from the sequence TAG of the inverted duplication. This might be the target sequence of plant telomere synthesis. It is noteworthy that a strange sequence, TATATA, was inserted in the telomere arrays. Similar sequences were also present in the inverted duplication sequence. This sequence may have acted with a specific function in the telomere synthesis.
The DNA structure at the break points of the other deletion lines will elucidate the contrivance of healing and the manner of the de novo synthesis of the telomere repeats.
Acknowledgments
We thank Dr. T. R. Endo (Kyoto University, Japan) for giving us the deletion lines, Dr. N. Kawakami and Mr. K. Nagaki in our laboratory for instructing us in DNA sequencing and molecular techniques, and Dr. H. S. Dhaliwal, Punjab Agricultural University, for critical reading and useful discussion of this manuscript. This work was partly supported by Grant-in-Aid 07740586 from the Ministry of Education, Science, Sports and Culture, Japan, and by a grant from the Kanagawa Academy of Science and Technology.
ABBREVIATIONS
- BFB
breakage–fusion–bridge
- NOR
nucleolar organizing region
- rDNA
rRNA gene
- CS
Chinese Spring
Footnotes
References
- 1.McClintock B. Genetics. 1941;26:234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkie A O M, Lamb J, Harris P C, Finney D R, Higgs D R. Nature (London) 1990;346:868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto H, Tsunewaki K. Jpn J Genet. 1985;60:565–578. [Google Scholar]
- 4.Tsujimoto H, Tsunewaki K. Can J Genet Cytol. 1985;27:178–185. [Google Scholar]
- 5.Tsujimoto H, Noda K. Wheat Inf Serv. 1990;71:6–9. [Google Scholar]
- 6.Endo T R, Gill B S. J Hered. 1996;87:295–307. [Google Scholar]
- 7.Tsujimoto H, Noda K. Genome. 1989;32:1085–1090. [Google Scholar]
- 8.Tsujimoto H, Noda K. Genome. 1990;33:850–853. [Google Scholar]
- 9.Werner J, Endo T R, Gill B S. Proc Natl Acad Sci USA. 1992;89:11307–11311. doi: 10.1073/pnas.89.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kota R S, Gill K S, Gill B S, Endo T R. Genome. 1993;36:548–554. doi: 10.1139/g93-075. [DOI] [PubMed] [Google Scholar]
- 11.Ogihara Y, Hasegawa K, Tsujimoto H. Mol Gen Genet. 1994;244:253–259. doi: 10.1007/BF00285452. [DOI] [PubMed] [Google Scholar]
- 12.Werner J, Kota R S, Gill B S, Endo T R. Genome. 1992;35:844–848. [Google Scholar]
- 13.Tsujimoto H. J Plant Res. 1993;106:239–244. [Google Scholar]
- 14.Gerlach W L, Bedbrook J R. Nucleic Acids Res. 1979;7:1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill B S, Friebe B, Endo T R. Genome. 1991;34:830–839. [Google Scholar]
- 16.Endo T R. J Hered. 1988;79:366–370. doi: 10.1093/oxfordjournals.jhered.a110529. [DOI] [PubMed] [Google Scholar]
- 17.Mukai Y, Endo T R, Gill B S. Chromosoma. 1991;100:71–78. [Google Scholar]
- 18.Tsujimoto H, Niwa K. Jpn J Genet. 1992;67:233–241. [Google Scholar]
- 19.Appels R, Moran L B. Stadler Genet Symp Ser. 1984;16:509–558. [Google Scholar]
- 20.Koebner R M D, Appels R, Shepherd K W. Can J Genet Cytol. 1986;28:658–664. [Google Scholar]
- 21.Barker R F, Harberd N P, Jarvis M G, Flavell R B. J Mol Biol. 1988;201:1–17. doi: 10.1016/0022-2836(88)90434-2. [DOI] [PubMed] [Google Scholar]
- 22.Kiss T, Kiss M, Solymosy F. Nucleic Acids Res. 1989;17:796. doi: 10.1093/nar/17.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiss T, Kiss M, Abel S, Solymosy F. Nucleic Acids Res. 1989;17:2127. doi: 10.1093/nar/17.5.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards E J, Ausubel F M. Cell. 1988;53:127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- 25.D’Ovidio R, Proceddu E, Lafiandra D. Theor Appl Genet. 1994;88:175–180. doi: 10.1007/BF00225894. [DOI] [PubMed] [Google Scholar]
- 26.Burr B, Burr F A, Matz E C, Romero-Severson J. Plant Cell. 1992;4:953–960. doi: 10.1105/tpc.4.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilian A, Kleinhofs A. Mol Gen Genet. 1992;86:705–712. doi: 10.1007/BF00286193. [DOI] [PubMed] [Google Scholar]
- 28.Cooke H. In: Telomere. Blackburn E H, Greider C W, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. pp. 219–245. [Google Scholar]
- 29.Hohmann U, Endo T R, Herrmann R G, Gill B S. Theor Appl Genet. 1995;91:611–617. doi: 10.1007/BF00223287. [DOI] [PubMed] [Google Scholar]
- 30.Lukaszewski A J. Genetics. 1995;140:1069–1085. doi: 10.1093/genetics/140.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo T R. Jpn J Genet. 1990;65:135–152. [Google Scholar]
- 32.Röder M S, Lapitan N L V, Sorrels M E, Tanksley S D. Mol Gen Genet. 1993;238:294–303. doi: 10.1007/BF00279558. [DOI] [PubMed] [Google Scholar]
- 33.Lamb J, Harris P C, Wilkie A O, Wood W G, Dauwerse J G, Higgs D R. Am J Hum Genet. 1993;52:668–676. [PMC free article] [PubMed] [Google Scholar]