Abstract
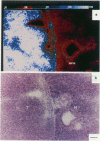
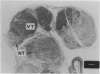
Experimental studies have been carried out using 5-aminolaevulinic acid (ALA) to induce transient porphyrin photosensitisation for photodynamic therapy (PDT) in a pancreatic cancer model in Syrian golden hamsters. ALA was given either intravenously or orally (in bolus or fractionated doses) with the laser light delivered by means of a bare fibre touching the tissue surface or external irradiation using a light-integrating cylindrical applicator. Animals were killed 1-24 h after ALA administration for pharmacokinetic studies and 3-7 days after light exposure to study PDT-induced necrosis. A separate survival study was also performed after a fractionated oral dose of ALA and external irradiation. Protoporphyrin IX sensitisation in the tumour tissue as measured by quantitative fluorescence microscopy was highest after intravenous administration of 200 mg kg-1 ALA and then in decreasing order after oral fractionated and oral bolus doses (both 400 mg kg-1). Laser light application at 630 nm to give 12-50 J from the bare fibre or 50 J cm-2 using surface illumination with the cylindrical applicator resulted in tumour necrosis up to 8 mm in depth. In larger tumours a rim of viable tumour was observed on the side opposite to illumination. In a randomised study, survival of treated animals was significantly longer than in the untreated control group (log-rank test, P < 0.02), although all animals died of recurrent tumour. This technique shows promise in the treatment of small volumes of tumour in the pancreas.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLIN N. I., NEUBERGER A., SCOTT J. J. The metabolism of delta -aminolaevulic acid. 1. Normal pathways, studied with the aid of 15N. Biochem J. 1956 Sep;64(1):80–90. doi: 10.1042/bj0640080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr H., Tralau C. J., MacRobert A. J., Krasner N., Boulos P. B., Clark C. G., Bown S. G. Photodynamic therapy in the normal rat colon with phthalocyanine sensitisation. Br J Cancer. 1987 Aug;56(2):111–118. doi: 10.1038/bjc.1987.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell J., MacRobert A. J., Phillips D., Bown S. G. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br J Cancer. 1992 Jun;65(6):818–824. doi: 10.1038/bjc.1992.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown S. G. Photodynamic therapy to scientists and clinicians--one world or two? J Photochem Photobiol B. 1990 Jun;6(1-2):1–12. doi: 10.1016/1011-1344(90)85069-9. [DOI] [PubMed] [Google Scholar]
- Chatlani P. T., Nuutinen P. J., Toda N., Barr H., MacRobert A. J., Bedwell J., Bown S. G. Selective necrosis in hamster pancreatic tumours using photodynamic therapy with phthalocyanine photosensitization. Br J Surg. 1992 Aug;79(8):786–790. doi: 10.1002/bjs.1800790826. [DOI] [PubMed] [Google Scholar]
- DeLaney T. F., Sindelar W. F., Tochner Z., Smith P. D., Friauf W. S., Thomas G., Dachowski L., Cole J. W., Steinberg S. M., Glatstein E. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int J Radiat Oncol Biol Phys. 1993 Feb 15;25(3):445–457. doi: 10.1016/0360-3016(93)90066-5. [DOI] [PubMed] [Google Scholar]
- Divaris D. X., Kennedy J. C., Pottier R. H. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am J Pathol. 1990 Apr;136(4):891–897. [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Cooper M. T., Mang T. S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med. 1990;10(5):485–488. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- Egami H., Takiyama Y., Cano M., Houser W. H., Pour P. M. Establishment of hamster pancreatic ductal carcinoma cell line (PC-1) producing blood group-related antigens. Carcinogenesis. 1989 May;10(5):861–869. doi: 10.1093/carcin/10.5.861. [DOI] [PubMed] [Google Scholar]
- Evrard S., Aprahamian M., Marescaux J. Intra-abdominal photodynamic therapy: from theory to feasibility. Br J Surg. 1993 Mar;80(3):298–303. doi: 10.1002/bjs.1800800309. [DOI] [PubMed] [Google Scholar]
- Grant W. E., Hopper C., MacRobert A. J., Speight P. M., Bown S. G. Photodynamic therapy of oral cancer: photosensitisation with systemic aminolaevulinic acid. Lancet. 1993 Jul 17;342(8864):147–148. doi: 10.1016/0140-6736(93)91347-o. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992 Jul 30;14(4):275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- Loh C. S., MacRobert A. J., Bedwell J., Regula J., Krasner N., Bown S. G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer. 1993 Jul;68(1):41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C. S., Vernon D., MacRobert A. J., Bedwell J., Bown S. G., Brown S. B. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J Photochem Photobiol B. 1993 Sep;20(1):47–54. doi: 10.1016/1011-1344(93)80130-2. [DOI] [PubMed] [Google Scholar]
- Malik Z., Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer. 1987 Nov;56(5):589–595. doi: 10.1038/bjc.1987.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang T. S., Wieman T. J. Photodynamic therapy in the treatment of pancreatic carcinoma: dihematoporphyrin ether uptake and photobleaching kinetics. Photochem Photobiol. 1987 Nov;46(5):853–858. doi: 10.1111/j.1751-1097.1987.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Cui Z. J. Photodynamic action of sulphonated aluminium phthalocyanine (SALPC) on AR4-2J cells, a carcinoma cell line of rat exocrine pancreas. Br J Cancer. 1990 May;61(5):695–701. doi: 10.1038/bjc.1990.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustajoki P., Timonen K., Gorchein A., Seppäläinen A. M., Matikainen E., Tenhunen R. Sustained high plasma 5-aminolaevulinic acid concentration in a volunteer: no porphyric symptoms. Eur J Clin Invest. 1992 Jun;22(6):407–411. doi: 10.1111/j.1365-2362.1992.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Nuutinen P. J., Chatlani P. T., Bedwell J., MacRobert A. J., Phillips D., Bown S. G. Distribution and photodynamic effect of disulphonated aluminium phthalocyanine in the pancreas and adjacent tissues in the Syrian golden hamster. Br J Cancer. 1991 Dec;64(6):1108–1115. doi: 10.1038/bjc.1991.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Moan J., Warloe T., Nesland J. M., Rimington C. Distribution and photosensitizing efficiency of porphyrins induced by application of exogenous 5-aminolevulinic acid in mice bearing mammary carcinoma. Int J Cancer. 1992 Sep 30;52(3):433–443. doi: 10.1002/ijc.2910520318. [DOI] [PubMed] [Google Scholar]
- Schoenfeld N., Epstein O., Lahav M., Mamet R., Shaklai M., Atsmon A. The heme biosynthetic pathway in lymphocytes of patients with malignant lymphoproliferative disorders. Cancer Lett. 1988 Dec 1;43(1-2):43–48. doi: 10.1016/0304-3835(88)90211-x. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Egami H., Pour P. M. Expression of human tumor-associated antigens in pancreatic cancer induced in Syrian hamsters. Am J Pathol. 1990 Mar;136(3):707–715. [PMC free article] [PubMed] [Google Scholar]
- Tralau C. J., Young A. R., Walker N. P., Vernon D. I., MacRobert A. J., Brown S. B., Bown S. G. Mouse skin photosensitivity with dihaematoporphyrin ether (DHE) and aluminium sulphonated phthalocyanine (AlSPc): a comparative study. Photochem Photobiol. 1989 Mar;49(3):305–312. doi: 10.1111/j.1751-1097.1989.tb04111.x. [DOI] [PubMed] [Google Scholar]
- Van Hillegersberg R., Van den Berg J. W., Kort W. J., Terpstra O. T., Wilson J. H. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology. 1992 Aug;103(2):647–651. doi: 10.1016/0016-5085(92)90860-2. [DOI] [PubMed] [Google Scholar]