Abstract
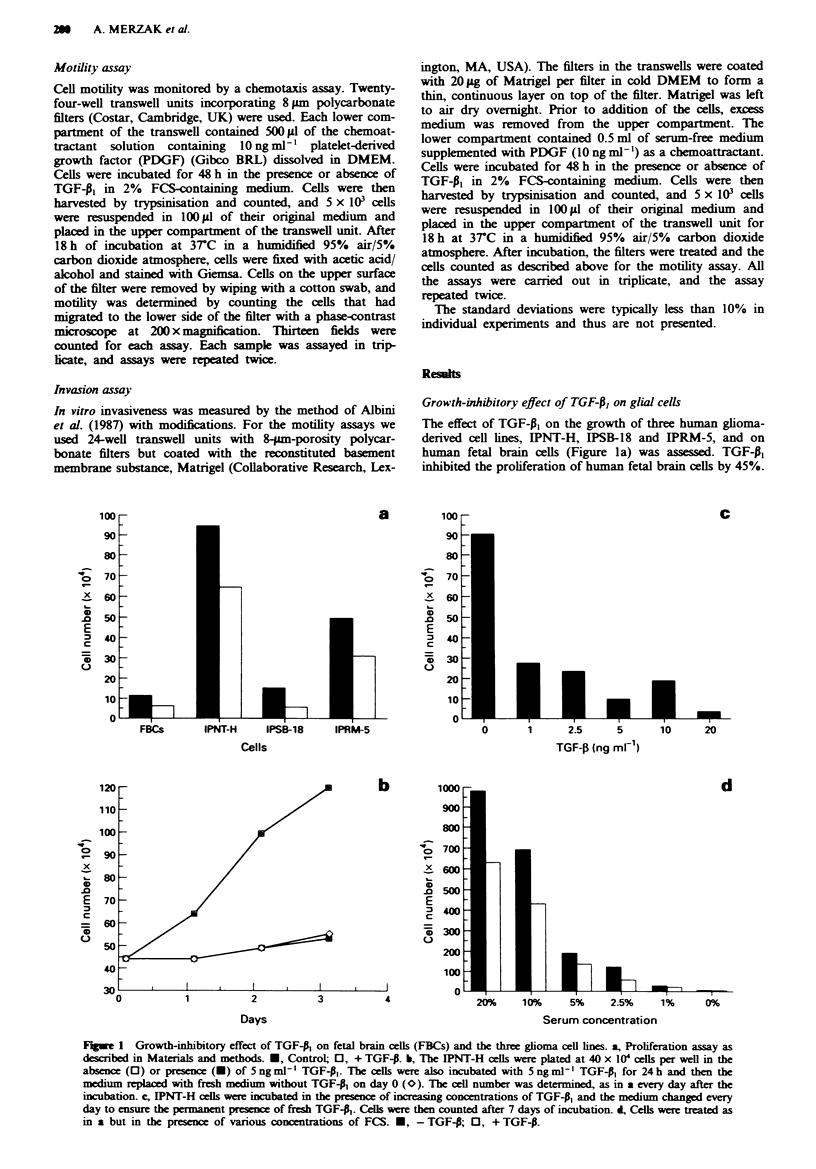
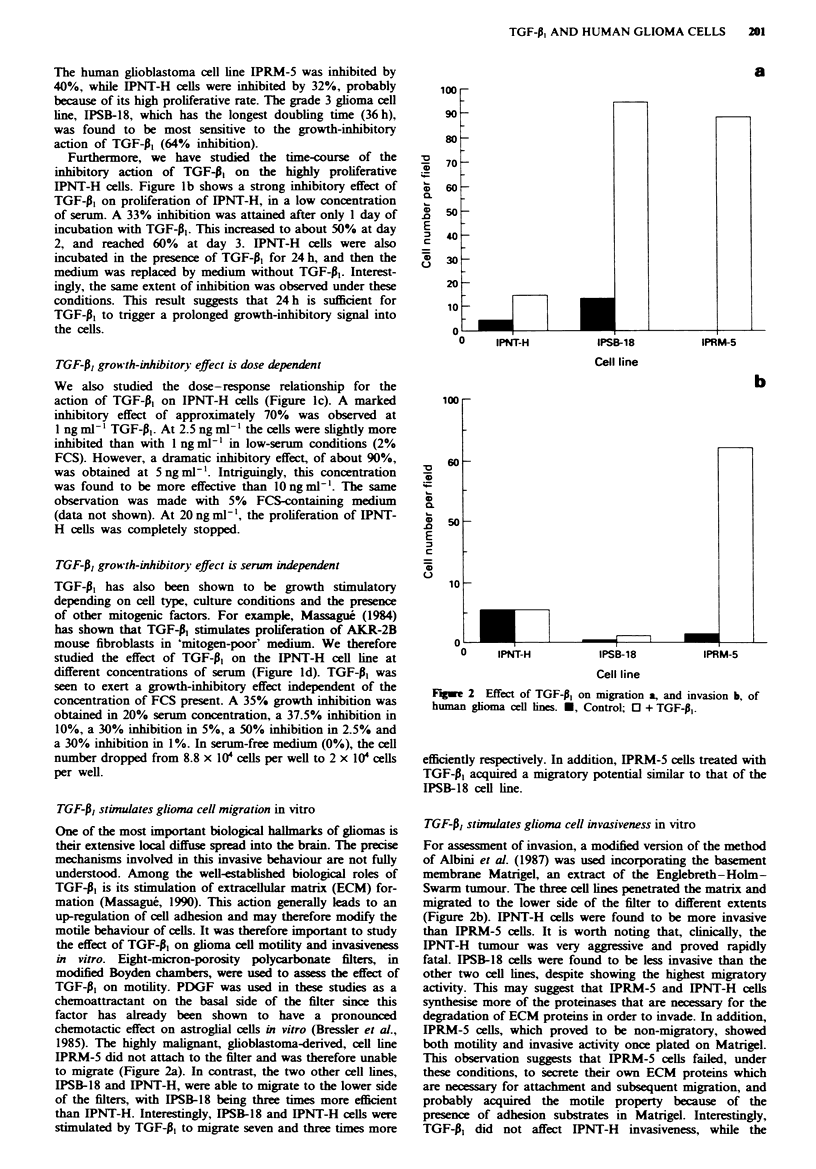
Factors involved in the control of the biological properties of gliomas, the major form of brain tumour in man, are poorly documented. We investigated the role of transforming growth factor beta 1 (TGF-beta 1) in the control of proliferation of human glioma cell lines as well as normal human fetal brain cells. The data presented show that TGF-beta 1 exerts a growth-inhibitory action on both human fetal brain cells and three cell lines derived from human glioma of different grades of malignancy. In addition, this growth-inhibitory effect is dose dependent and serum independent. Since TGF-beta 1 is known to be involved in the control of cell migration during ontogenesis and oncogenesis, we investigated the role of this factor in the motile and invasive behaviour that characterises human gliomas in vivo. TGF-beta 1 was found to elicit a strong stimulation of migration and invasiveness of glioma cells in vitro. In combination with recent data showing an inverse correlation between TGF-beta 1 expression in human gliomas and survival, these findings may suggest that TGF-beta 1 plays an important role in the malignant progression of gliomas in man. A study of the molecular mechanisms involved in the antiproliferative action and the invasion-promoting action of TGF-beta 1 may help to identify new targets in therapy for brain tumours. A combined antiproliferative and anti-invasive therapy could be envisaged.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Basler K., Edlund T., Jessell T. M., Yamada T. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993 May 21;73(4):687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Bressler J. P., Grotendorst G. R., Levitov C., Hjelmeland L. M. Chemotaxis of rat brain astrocytes to platelet derived growth factor. Brain Res. 1985 Oct 7;344(2):249–254. doi: 10.1016/0006-8993(85)90802-9. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Structure, function and biological properties of integrin alpha v beta 3 on human melanoma cells. Cancer Metastasis Rev. 1991 May;10(1):3–10. doi: 10.1007/BF00046839. [DOI] [PubMed] [Google Scholar]
- Constam D. B., Philipp J., Malipiero U. V., ten Dijke P., Schachner M., Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992 Mar 1;148(5):1404–1410. [PubMed] [Google Scholar]
- Ito S., Hoshino T., Shibuya M., Prados M. D., Edwards M. S., Davis R. L. Proliferative characteristics of juvenile pilocytic astrocytomas determined by bromodeoxyuridine labeling. Neurosurgery. 1992 Sep;31(3):413–419. doi: 10.1227/00006123-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Rogers L. R., Barnett G. H., Estes M. L., Barna B. P. Effect of recombinant tumor necrosis factor-alpha on three-dimensional growth, morphology, and invasiveness of human glioblastoma cells in vitro. J Neurosurg. 1993 Jun;78(6):952–958. doi: 10.3171/jns.1993.78.6.0952. [DOI] [PubMed] [Google Scholar]
- Knott J. C., Edwards A. J., Gullan R. W., Clarke T. M., Pilkington G. J. A human glioma cell line retaining expression of GFAP and gangliosides, recognized by A2B5 and LB1 antibodies, after prolonged passage. Neuropathol Appl Neurobiol. 1990 Dec;16(6):489–500. doi: 10.1111/j.1365-2990.1990.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Labourdette G., Janet T., Laeng P., Perraud F., Lawrence D., Pettmann B. Transforming growth factor type beta 1 modulates the effects of basic fibroblast growth factor on growth and phenotypic expression of rat astroblasts in vitro. J Cell Physiol. 1990 Sep;144(3):473–484. doi: 10.1002/jcp.1041440315. [DOI] [PubMed] [Google Scholar]
- Lindholm D., Castrén E., Kiefer R., Zafra F., Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992 Apr;117(2):395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massagué J. Type beta transforming growth factor from feline sarcoma virus-transformed rat cells. Isolation and biological properties. J Biol Chem. 1984 Aug 10;259(15):9756–9761. [PubMed] [Google Scholar]
- McKinnon R. D., Piras G., Ida J. A., Jr, Dubois-Dalcq M. A role for TGF-beta in oligodendrocyte differentiation. J Cell Biol. 1993 Jun;121(6):1397–1407. doi: 10.1083/jcb.121.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Rawson C., Lindburg K., Barnes D. Serum and transforming growth factor beta regulate glial fibrillary acidic protein in serum-free-derived mouse embryo cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8378–8382. doi: 10.1073/pnas.87.21.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Derynck R. Developmental expression of transforming growth factors alpha and beta in mouse fetus. Mol Cell Biol. 1988 Aug;8(8):3415–3422. doi: 10.1128/mcb.8.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox J. N., Derynck R. Localization of cells synthesizing transforming growth factor-alpha mRNA in the mouse brain. J Neurosci. 1988 Jun;8(6):1901–1904. doi: 10.1523/JNEUROSCI.08-06-01901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martin R., Haendler B., Hofer-Warbinek R., Gaugitsch H., Wrann M., Schlüsener H., Seifert J. M., Bodmer S., Fontana A., Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987 Dec 1;6(12):3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



