Abstract
Protein kinase D (PKD; also known as PKCµ) is a serine/threonine kinase activated by diacylglycerol signalling pathways in a variety of cells. PKD has been described previously as Golgi-localized, but herein we show that it is present within the cytosol of quiescent B cells and mast cells and moves rapidly to the plasma membrane after antigen receptor triggering. The membrane redistribution of PKD requires the diacylglycerol-binding domain of the enzyme, but is independent of its catalytic activity and does not require the integrity of the pleckstrin homology domain. Antigen receptor signalling initiates in glycosphingolipid-enriched microdomains, but membrane-associated PKD does not co-localize with these specialized structures. Membrane targeting of PKD is transient, the enzyme returns to the cytosol within 10 min of antigen receptor engagement. Strikingly, the membrane-recycled PKD remains active in the cytosol for several hours. The present work thus characterizes a sustained antigen receptor-induced signal transduction pathway and establishes PKD as a serine kinase that temporally and spatially disseminates antigen receptor signals away from the plasma membrane into the cytosol.
Keywords: antigen receptor/cysteine-rich domain/diacylglycerol/PKCµ/PKD
Introduction
Antigen receptors are critical regulators of lymphocyte function. Antigen receptor signal transduction is mediated directly by tyrosine kinases that control a diverse network of signalling molecules, including enzymes involved in the control of inositol phospholipid metabolism such as phospholipase Cγ (PLCγ). The activation of PLCγ induces phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] hydrolysis, producing IP3 and diacylglycerol (DAG), which regulate intracellular calcium and activate the protein kinase C (PKC) family of serine/threonine kinases, respectively. There are multiple PKC isoforms, including classical PKCs (α, βI, βII and γ), which bind calcium, DAG and phospholipids; novel PKCs (δ, ε, η and θ), which are regulated by DAG and phospholipids; and the atypical PKCs (ξ and λ), which lack calcium- or DAG-binding domains (Newton, 1997; Mellor and Parker, 1998; Toker, 1998). The significance and potency of calcium and PKC signalling pathways for lymphocyte activation have been well documented (Berry and Nishizuka, 1990; Crabtree and Clipstone, 1994; Leitges et al., 1996; Crabtree, 1999).
Recently, it has been demonstrated that antigen receptors stimulate the activity of protein kinase D (PKD; also known as PKCµ) (Sidorenko et al., 1996; Matthews et al., 1999). However, despite the clear functional link between PKD and antigen receptors, there has been no analysis of PKD localization in resting or activated lymphocytes. It has been reported that PKD functions to regulate Golgi organization and transport processes (Prestle et al., 1996; Jamora et al., 1999). It has also been described that PKD is localized partially with the Golgi compartment in a human hepatocellular carcinoma cell line and in human antral gastrin cells (Prestle et al., 1996; Moore et al., 1999). However, there has never been any integration of data about PKD activity and localization under physiological conditions of cell activation. In this context, the cellular activation of PKD is mediated by PKCs, which require lipid co-activators for catalytic activity, thereby restricting their activity to lipid-enriched subcellular locations, typically the plasma membrane. In contrast, PKD catalytic activity is regulated by protein phosphorylation (Iglesias et al., 1998b) and can be maintained in the absence of lipid co-activators (Zugaza et al., 1996, 1997; Matthews et al., 1997; Van Lint et al., 1998). PKD thus has the potential to be active away from membrane/lipophilic environments and could be active in the cytosol.
One feature of PKD that might determine its subcellular localization is the presence of a conserved DAG-binding cysteine-rich domain (CRD) within its regulatory region that has previously been demonstrated to bind DAG and phorbol esters (Valverde et al., 1994; Van Lint et al., 1995; Dieterich et al., 1996). Classical and novel PKCs possess a CRD motif, which plays a critical role in recruiting these enzymes to the plasma membrane of various epithelial and fibroblast cell lines (Sakai et al., 1997; Feng et al., 1998; Oancea et al., 1998; Ohamori et al., 1998). CRD motifs additionally may target PKCs to other intracellular membranes, including the nuclear envelope (Oancea and Meyer, 1998; Oancea et al., 1998; Ohamori et al., 1998). However, the presence of a CRD domain is not necessarily sufficient for membrane targeting: recruitment of classical PKCs to the plasma membrane requires the binding of both DAG and calcium to regulatory CRD and C2 domains, respectively (Oancea and Meyer, 1998). Moreover, there are clearly other regulatory factors that can override the presence of a CRD domain to determine protein localization. Novel PKCs all have a CRD domain, but only θ is recruited to the plasma membrane during antigenic stimulation of T cells (Monks et al., 1997, 1998). In this context, the N-terminal regulatory region of PKD does not possess the calcium-binding C2 domain essential for effective membrane targeting of classical PKCs. PKD does, however, contain a putative pleckstrin homology (PH) domain (Valverde et al., 1994), which has the potential to play an important role in controlling the subcellular localization of PKD either through binding to lipid second messengers or via protein–protein interactions (Pitcher et al., 1995; Leevers et al., 1999; Lemmon, 1999).
The objective of the present report was to use biochemical analysis and real-time confocal imaging of green fluorescent protein (GFP)-tagged PKD molecules in living cells to explore the localization of PKD in antigen receptor-activated cells. Importantly, this study integrates spatial and temporal aspects of PKD localization with PKD catalytic activity under physiological conditions of cell activation. Our results identify PKD as a signalling molecule that has two sites of action in lymphocytes: initially, PKD operates at the plasma membrane outside lipid microdomains during the initial activation response, but functions within the cytosol during sustained responses to antigen receptor ligation.
Results
Activation of PKD in B lymphocytes and in mast cells following antigen receptor ligation or phorbol ester activation
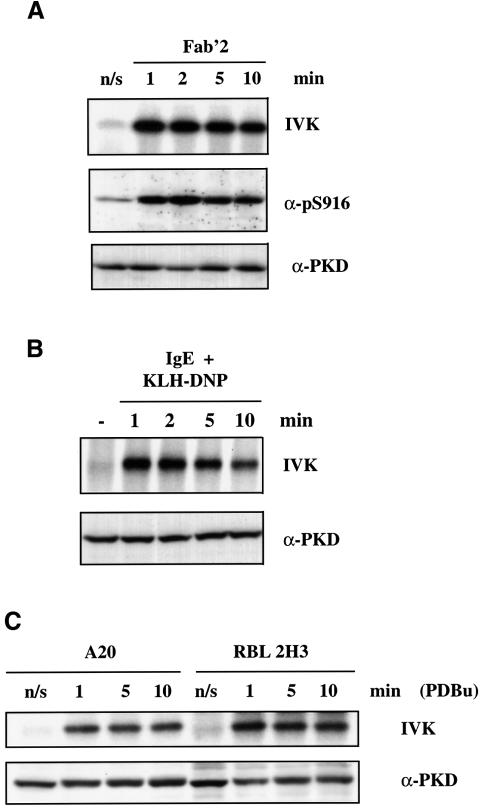
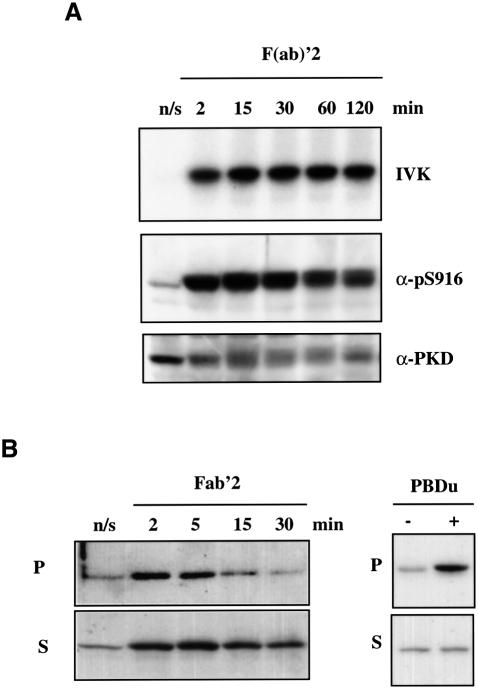
A20 B-lymphoma cells were activated by cross-linking the B-cell antigen receptor (BCR) with F(ab)′2 fragments of anti-mouse IgG for various times (0–10 min). Cross-linking of the BCR complex was found to induce a rapid ∼7- to 8-fold increase in PKD catalytic activity (Figure 1A), a response that was maximal within 1 min of BCR triggering. Active PKD autophosphorylates on a C-terminal serine residue, S916 (Matthews et al., 1999), and antibodies that recognize S916-phosphorylated PKD can be used to monitor the activity of PKD. As shown in Figure 1A, the pS916 antiserum only weakly recognized PKD from unstimulated cells, but reacted strongly with active PKD isolated from BCR-activated A20 B lymphocytes. The antigen receptor present in mast cells is the high affinity receptor for IgE, the FcεR1. The data presented in Figure 1B indicate that activation of the FcεR1 induces a rapid ∼6-fold increase in PKD catalytic activity.
Fig. 1. BCR or FcεR1 ligation induces PKD catalytic activity. (A) A20 B lymphocytes were left untreated (n/s) or were stimulated with 10 µg/ml F(ab)′2 fragments of anti-mouse IgG for various times (0–10 min). The cells were subsequently lysed before PKD was immunoprecipitated using the PA-1 antiserum and in vitro kinase assays performed. PKD activity was measured by autophosphorylation (IVK). Alternatively, total proteins from cell lysates were precipitated with cold acetone, separated by SDS–PAGE and subjected to western blot analysis using the pS916 antiserum and a pan C-terminal PKD antibody. Results are representative of three independent experiments. (B) RBL 2H3 cells were primed with 1 µg/ml IgE anti-DNP for 1 h at 37°C and then either antigenic cross-linking of the bound IgE was performed, using 500 ng/ml KLH-DNP, for various times (0–10 min) or the cells were left unstimulated (–). PKD was immunoprecipitated from cell lysates and the activity of PKD was measured by in vitro kinase assays (IVK). SDS–PAGE and western blot analysis of the cell lysates show equivalent amounts of PKD in all the samples. Results are representative of two independent experiments. (C) RBL 2H3 or A20 B cells were either left unstimulated (n/s) or were stimulated with 50 ng/ml PDBu for various times (0–10 min). PKD was immunoprecipitated from cell lysates and the activity of PKD was measured by in vitro kinase assays (IVK). Western blot analysis of the cell lysates shows equivalent amounts of PKD in all the samples. Results are representative of two independent experiments.
The effects of stimulating B lymphocytes and mast cells with the phorbol ester phorbol 12,13 dibutyrate (PDBu), a pharmacological activator of PKC enzymes, on PKD activity were also measured. As shown in Figure 1C, PDBu treatment led to a rapid increase in the catalytic activity of PKD in both cell types. The kinetics and magnitude of this response (reaching a maximum 6- to 7-fold increase in PKD activity within ∼1 min) were essentially identical to those observed upon engagement of antigen receptors in these cells.
Cellular localization of inactive PKD in resting mast cells and B lymphocytes
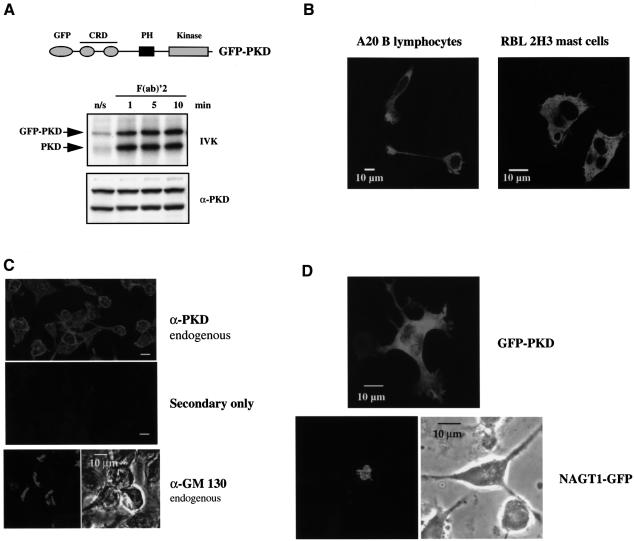
To explore the spatial dynamics of PKD regulation in lymphocytes, a GFP-tagged version of wild-type PKD (GFP–PKD) was generated and expressed in either A20 B lymphocytes or the RBL 2H3 mast cell line. Addition of a GFP tag to the N-terminus of PKD did not affect basal PKD activity nor did it prevent antigen receptor-induced activation of PKD in B lymphocytes (Figure 2A) or in RBL 2H3 mast cells (data not shown). The localization of GFP-tagged PKD in quiescent and antigen receptor-activated cells was then analysed. The data in Figure 2B show mid-section confocal images taken from RBL 2H3 cells or from A20 B lymphocytes that had been transiently transfected with GFP–PKD. These images indicate that GFP–PKD was distributed evenly throughout the cytosol of these cells, with no apparent association with specific intracellular compartments, and was excluded from the nucleus. Importantly, immunofluorescence staining of fixed cells with a specific polyclonal PKD antibody confirmed that the endogenous PKD present in resting A20 B lymphocytes was distributed evenly throughout the cytosol of these cells (Figure 2C). It should be emphasized that the C-terminal epitope recognized by this antibody is unique to PKD and does not cross-react with the recently described serine kinase, PKCυ, which exhibits strong homology to PKD (Hayashi et al., 1999). The cytosolic distribution of PKD in B lymphocytes and mast cells demonstrated herein contrasts with the reported Golgi localization of PKD described in a hepatocellular carcinoma cell line and in human antral gastrin cells (Prestle et al., 1996; Moore et al., 1999). In further experiments, we noted that PKD localization in A20 B lymphocytes was clearly distinct from that of endogenous GM 130 (Figure 2C) or ectopically overexpressed GFP-tagged NAGT1 (Figure 2D), two well characterized Golgi-localized proteins (Nakamura et al., 1995; Shima et al., 1997). The data in Figure 2D show a comparison of the subcellular distribution of GFP–PKD or NAGT1–GFP in A20 B lymphocytes. Two-dimensional projections of sections in Z-series taken across the depth of the cells at 0.3 µm intervals clearly reveal the distinct pattern of PKD localization compared with this Golgi marker, demonstrating that PKD is not localized preferentially to the Golgi in B lymphocytes or in mast cells.
Fig. 2. Localization of PKD in resting A20 B lymphocytes and RBL 2H3 cells. (A) Top: schematic representation of the N-terminally GFP-tagged PKD construct. Bottom: A20 B lymphocytes were transiently transfected with 20 µg of wild-type GFP–PKD and left to recover overnight. Cells were left untreated (n/s) or were stimulated with 10 µg/ml F(ab)′2 fragments of anti-mouse IgG for various times (1–10 min). The cells were subsequently lysed before PKD (endogenous) and GFP–PKD (ectopically expressed) were immunoprecipitated using the PA-1 antiserum and in vitro kinase assays performed. PKD activity was measured by autophosphorylation (IVK). Western blot analysis of whole-cell lysates with a specific PKD antibody reveals PKD expression levels. Similar results were obtained in two independent experiments. (B) A20 B lymphocytes or RBL 2H3 mast cells were transiently transfected overnight with 10 µg of GFP–PKD cDNA. Shown are mid-section confocal images showing the expression of GFP–PKD in these cells. (C) Immunofluorescence staining of A20 B lymphocytes. Top: cells stained with a specific C-terminal PKD antibody (sc-935) to reveal the subcellular distribution of endogenous PKD. Middle: cells stained with secondary layer only. Bottom: immunofluorescence staining of the endogenous GM 130 present in A20 B lymphocytes using a GM 130 polyclonal antibody (Nakamura et al., 1995). Bar = 10 µm. (D) Comparison of the subcellular distribution of exogenously expressed GFP–PKD or NAGT1–GFP in A20 B lymphocytes. Images represent two-dimensional projections of sections in Z-series taken across the depth of the cells at 0.3 µm intervals.
Antigen receptor ligation induces a transient relocalization of PKD to the plasma membrane
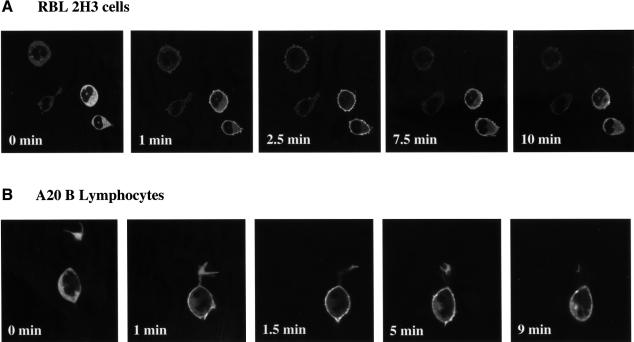
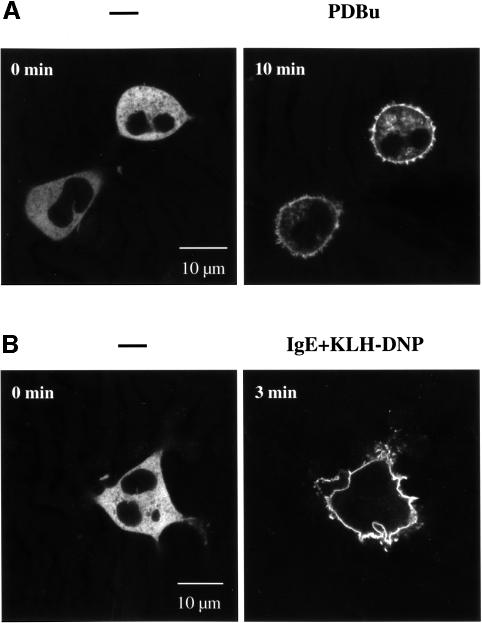
To examine the effect of antigen receptor ligation on localization of PKD, RBL 2H3 cells expressing GFP–PKD were activated via the FcεR1 and confocal images were taken of live cells in real time. The data (Figure 3A) demonstrate that activation of the FcεR1 induces a rapid redistribution of GFP–PKD from the cytosol to the plasma membrane of mast cells. Membrane targeting of GFP–PKD was detectable <1 min after FcεR1 ligation of RBL 2H3 cells and was maximal within ∼1–2 min (Figure 3A). The effect of antigen receptor ligation on PKD localization was also investigated in A20 B lymphocytes. Stimulation of A20 B lymphocytes with F(ab)′2 fragments was found to induce a rapid translocation of GFP–PKD from the cytosol to the plasma membrane of these cells within ∼1–2 min of BCR activation (Figure 3B).
Fig. 3. Antigen receptor ligation induces a rapid but transient association of GFP–PKD with the plasma membrane. RBL 2H3 cells (A) or A20 B lymphocytes (B), transiently expressing GFP–PKD, were imaged in real time before and after FcεR1 or BCR ligation, respectively. GFP–PKD rapidly translocated to the plasma membrane of antigen receptor-activated cells after antigen receptor engagement. The precise timing of GFP–PKD translocation varied from cell to cell, but consistently maximal plasma membrane localization of GFP–PKD was seen within 1–2 min of antigen receptor stimulation. GFP–PKD subsequently dissociated from the membrane, returning to the cytosol completely after ∼8–10 min of antigen receptor activation. Similar results were observed in the majority of B lymphocytes (>95%) and mast cells (>90%) observed. The results shown are representative of at least four independent experiments where each time 6–8 individual cells were imaged.
Strikingly, GFP–PKD association with the plasma membrane of antigen receptor-stimulated cells was transient. Time-lapse confocal microscopy clearly demonstrated that GFP–PKD consistently dissociated from the plasma membrane of FcεR1-activated mast cells (Figure 3A) or BCR-stimulated lymphocytes (Figure 3B) within ∼8–10 min of antigen receptor engagement.
PKD localization in phorbol ester-stimulated B lymphocytes and mast cells
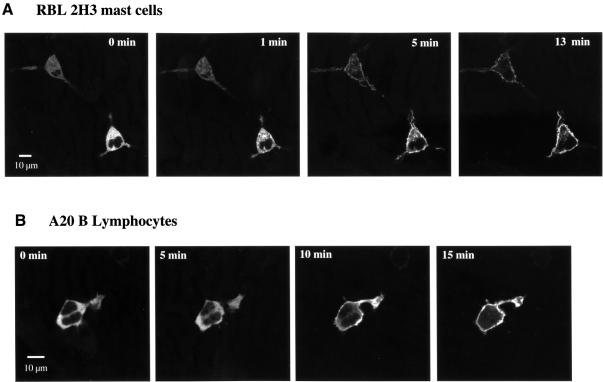
The data presented in Figure 4A demonstrate that the phorbol ester PDBu induces translocation of GFP–PKD to the plasma membrane of RBL 2H3 cells. Membrane targeting of GFP–PKD was detectable within ∼6–8 min of PDBu treatment of RBL 2H3 cells and was maximal within ∼10–15 min (Figure 4A). Stimulation of A20 B lymphocytes with PDBu was also found to induce complete translocation of GFP–PKD from the cytosol to the plasma membrane of these cells within ∼10 min (Figure 4B). Image analysis of these cells for longer time periods found that the association of PKD with the plasma membrane was maintained for several hours in the continued presence of PDBu, with no evidence for the redistribution of PKD back into the cytosol (data not shown). The stable nature of PKD membrane translocation following phorbol ester treatment was in marked contrast to the transient plasma membrane residence of PKD observed in cells activated by antigen receptor engagement.
Fig. 4. Redistribution of GFP–PKD to the plasma membrane in A20 B lymphocytes and RBL 2H3 mast cells in response to phorbol ester treatment. RBL 2H3 cells (A) or A20 B lymphocytes (B), transiently expressing GFP–PKD, were imaged in real time before and after the addition of 50 ng/ml PDBu, using an inverted confocal microscope, at 37°C. Images taken at the indicated times are shown, with treatment carried out after the zero time point image had been acquired. Similar results were obtained in four independent experiments where at least 4–5 individual cells were analysed per experiment. Similar kinetics of translocation were observed in four independent experiments, where 4–5 individual cells were analysed each time.
We also noted one other more subtle difference in PKD localization in PDBu-treated versus antigen receptor-activated RBL 2H3 cells. Here, PDBu stimulation caused the majority of PKD to redistribute to the plasma membrane. However, on close examination, a fraction of PKD was found localized to membranes surrounding cytoplasmic granules (Figure 5A). In contrast, FcεR1 stimulation of RBL 2H3 mast cells induced the total cellular pool of PKD to redistribute to the plasma membrane, with no detectable association with cytoplasmic granules (Figure 5B).
Fig. 5. Association of GFP–PKD with intracellular granules in phorbol ester- but not FcεR1-stimulated RBL 2H3 mast cells. RBL 2H3 mast cells, transiently expressing GFP–PKD, were imaged in real time before and after treatment with PDBu (A) or following FcεR1 ligation (B). PDBu induces association of GFP–PKD with both the plasma membrane and the membrane of cytosolic granules. In contrast, FcεR1 ligation specifically induces association of GFP–PKD with the plasma membrane. In all experiments, phorbol ester treatment gave a slow translocation to the plasma membrane while FcεR1 stimulation resulted in a rapid plasma membrane translocation. The number of experiments was six and four, respectively, where 4–5 individual cells were analysed in each experiment.
Sustained activation of PKD by antigen receptor engagement
PKD is thus localized transiently to the plasma membrane of B lymphocytes and mast cells following antigen receptor ligation, but subsequently returns to the cytosol in a dynamic cycle that is complete within 8–10 min following receptor stimulation. To examine whether the dissociation of PKD from the plasma membrane was accompanied by a reduction in its kinase activity, the kinetics of PKD activation were compared with those of PKD membrane targeting. The data presented in Figure 1 indicated that substantial PKD activity could be detected after 10 min of antigen receptor engagement in both B lymphocytes and mast cells, at times when PKD dissociation from the plasma membrane was complete (see Figure 3).
To investigate the long-term kinetics of PKD activation following antigen receptor ligation, A20 B lymphocytes were stimulated with F(ab)′2 for a period of up to 2 h. PKD activity was then measured at the indicated time points by in vitro kinase assays and by western blot analysis of total cell lysates using the pS916 antibody (which selectively recognizes active PKD). As Figure 6A indicates, BCR-induced activation of PKD in A20 B lymphocytes is an extremely sustained response: maximal activity was seen within 1 min of antigen receptor ligation and was maintained at high levels throughout the 10 min period that PKD is found associated with the plasma membrane. Importantly, both PKD catalytic activity and S916 phosphorylation remained high for at least 2 h post-receptor triggering, at times when PKD had fully dissociated from the plasma membrane (Figure 3 and data not shown).
Fig. 6. Sustained PKD activity following BCR ligation. (A) A20 B lymphocytes were plated in complete medium at a density of 1.5 × 107 cells/ml in a 24-well plate and left to recover at 37°C, 5% CO2 for 1 h before stimulation. Subsequently, the cells were left untreated (n/s) or were stimulated with 10 µg/ml F(ab)′2 fragments of anti-mouse IgG for various times (2–120 min). The cells were lysed and PKD immunoprecipitated using the PA-1 antiserum. In vitro kinase assays (IVK) were performed using PKD autophosphorylation as the measure of catalytic activity. Alternatively, proteins in the lysates were precipitated with cold acetone, separated by SDS–PAGE and subjected to western blot analysis using the pS916 antiserum and a pan C-terminal PKD antibody. Results are representative of three independent experiments. (B) A20 B lymphocytes were left untreated (n/s) or were stimulated with 10 µg/ml F(ab)′2 fragments of anti-mouse IgG for various times (0–30 min) or with 50 ng/ml PDBu for 15 min. Particulate (P; membrane) and soluble (S; cytosolic) fractions were prepared from the cells, as described in Materials and methods, and proteins in the resulting fractions were precipitated with cold acetone, separated by SDS–PAGE and subjected to western blot analysis using the pS916 antiserum to detect active PKD. Results are representative of two independent experiments.
The confocal images presented in Figure 3 indicated that active PKD does not localize to specific intracellular structures following its dissociation from the plasma membrane in antigen receptor-stimulated cells. We examined this further by biochemical fractionation experiments. As shown in Figure 6B, active PKD was found in the particulate (membrane) fraction of these cells within 2 min of BCR stimulation, but within ∼15 min the PKD activity within this compartment had diminished. In contrast, active PKD could be detected within the soluble (cytosolic) compartment of B lymphocytes up to 30 min after BCR stimulation, indicating that active PKD does not associate strongly with intracellular membranes following its dissociation from the plasma membrane. Importantly, PKD isolated from PDBu-treated B lymphocytes was active in the particulate fraction of these cells at these times (Figure 6B and data not shown), in agreement with the confocal data presented above demonstrating that PKD stably associates with the plasma membrane in response to phorbol ester treatment. Accordingly, these confocal images and biochemical data suggest that there are two phases of PKD regulation following antigen receptor stimulation in B lymphocytes and mast cells: an initial activation/recruitment to the plasma membrane followed by a sustained period of PKD activity within the cytosol.
Membrane targeting of GFP–PKD is mediated by the DAG-binding CRD
The structural basis for the membrane recruitment of a classical PKC isoform, PKCγ, has been explored in detail recently and requires the co-ordinate binding of calcium to the C2 domain followed by DAG binding to the CRD domain (Oancea and Meyer, 1998). The N-terminal regulatory region of PKD does not contain a C2 domain, but does contain a DAG-binding CRD domain and a putative PH domain (Valverde et al., 1994). To determine the relative contributions of the PH domain and the CRD to antigen receptor-induced PKD translocation, GFP-tagged versions of different PKD CRD or PH domain mutants were made. These were expressed transiently in A20 B lymphocytes, and a comparison of the distribution of these molecules in quiescent and BCR-stimulated cells was made by real-time confocal imaging of live cells.
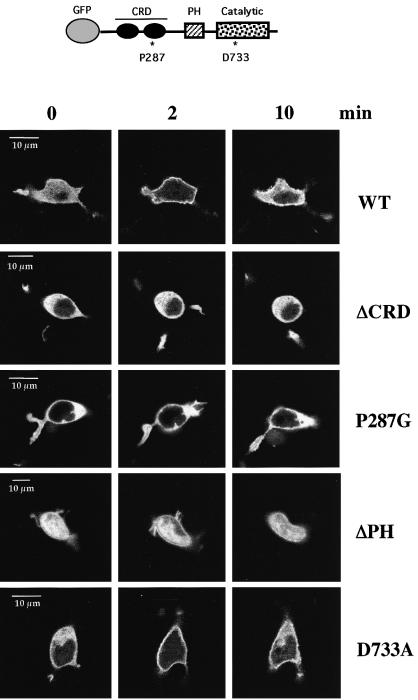
The GFP–PKD ΔCRD mutant contains a deletion of the entire CRD domain, whilst the GFP–PKD P287G mutant contains a proline to glycine substitution within the CRD. In both cases, the integrity of the CRD domain of PKD is destroyed and the binding of phorbol esters/DAG to PKD is prevented (Iglesias et al., 1998a). As shown in Figure 7, GFP–PKD ΔCRD and GFP–PKD P287G were distributed evenly throughout the cytosol of quiescent A20 cells and excluded from the nucleus, indistinguishably from wild-type GFP–PKD. Following BCR ligation, wild-type GFP–PKD transiently translocated from the cytosol to the plasma membrane, as observed previously. In contrast, real-time confocal imaging studies of cells showed that BCR ligation was repeatedly unable to induce the plasma membrane redistribution of either GFP–PKD ΔCRD or GFP–PKD P287G (Figure 7). Hence the BCR-induced translocation of PKD to the plasma membrane was dependent on the integrity of the DAG/phorbol ester-binding CRD domain of PKD.
Fig. 7. Plasma membrane targeting of GFP–PKD in response to BCR triggering is mediated by the DAG-binding CRD. A20 B lymphocytes were transiently transfected with different GFP–PKD mutations: a deletion of the CRD (ΔCRD); a point mutation within CRD (P287G); a deletion of the PH domain (ΔPH); and a kinase-deficient PKD mutant (D733A). Confocal images of representative living cells are shown at different time points before and after BCR ligation at the times indicated. Transient plasma membrane association of the kinase-dead GFP–PKD D733A and the GFP–PKD ΔPH mutants was observed following BCR ligation, comparable with wild-type GFP–PKD. The kinetics of the subcellular redistribution of the ΔPH and the D733A GFP–PKD mutants were similar to those of wild-type GFP–PKD, with maximal membrane association seen at ∼1–2 min before returning to the cytosol within ∼7–10 min in >80% of the cells analysed. The cytosolic localization of GFP–PKD ΔCRD and GFP–PKD P287G was not altered upon BCR ligation in >95% or >75% of the BCR-stimulated cells imaged, respectively. Translocation of GFP–PKD mutants was visualized in at least six independent experiments, where 4–6 individual cells were analysed before and during BCR stimulation in each experiment.
To determine whether the PH domain of PKD was involved in modulating its subcellular localization, A20 B lymphocytes were transiently transfected with a PKD mutant containing a deletion of the entire PH domain (GFP–PKD ΔPH). The confocal images in Figure 7 show a homogeneous distribution of GFP–PKD ΔPH throughout the cytosol and the nucleus of resting B lymphocytes, unlike wild-type or CRD mutants of PKD whose localization was restricted to the cytosolic compartment. In response to BCR stimulation, an accumulation of GFP–PKD ΔPH at the plasma membrane was observed with an accompanying depletion of the cytosolic pool of GFP–PKD ΔPH (Figure 7). The plasma membrane targeting of GFP–PKD ΔPH was transient. No apparent change in the level of the nuclear-localized GFP–PKD ΔPH was seen following BCR stimulation. These results indicate that the PH domain of PKD does not contribute to the redistribution of PKD to or from the plasma membrane during antigen receptor triggering.
The intrinsic catalytic activity of PKD was not required to regulate either the basal localization of PKD or its transient redistribution to the plasma membrane following activation of the BCR complex. Hence, as shown in Figure 7, a GFP-tagged kinase-deficient PKD mutant (GFP–PKD D733A) localized to the cytosol of resting cells and translocated to the plasma membrane following BCR activation in the same transient manner as that observed for wild-type PKD.
PKD does not localize predominantly to glycosphingolipid-enriched microdomains (GEMs)
A number of tyrosine kinases and adaptor molecules involved in antigen receptor signalling have been found localized to GEMs within the plasma membrane of B and T lymphocytes and mast cells. GEMs control the spatial organization of membrane-bound and cytosolic signalling molecules (Field et al., 1997; Stauffer and Meyer, 1997; Zhang et al., 1998; Sheets et al., 1999). Activated antigen receptors are themselves found within GEMs and it is in these specialized membrane microdomains that antigen receptor-induced signalling cascades are initiated (Field et al., 1997; Stauffer and Meyer, 1997; Montixi et al., 1998). The localization of DAG-regulated enzymes in relationship to GEMs is of interest because it has been proposed that GEMs are the site of inositol lipid metabolism; both PLCγ and PI(4,5)P2 are concentrated within these structures (Hope and Pike, 1996; Stauffer and Meyer, 1997; Xavier et al., 1998; Zhang et al., 1998). The location of GEMs within plasma membranes can be visualized by staining cells with a fluorescently labelled cholera toxin (CT) B subunit, which selectively binds to the GM1 glycolipid found associated with these membrane microdomains (Stauffer and Meyer, 1997). To investigate whether GFP–PKD was associating with GEMs following its redistribution to the plasma membrane, we compared the localization of GFP–PKD with the distribution pattern of membrane microdomains identified by CT B subunit staining.
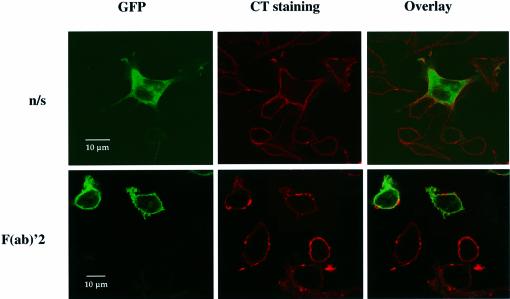
A20 B lymphocytes, expressing GFP–PKD, were left unstimulated or were stimulated briefly with F(ab)′2 before they were fixed and stained with biotinylated CT and streptavidin–Alexa568 conjugate. Confocal images of both the GFP and the CT signals were then obtained. Quiescent A20 B lymphocytes showed a uniform distribution of CT staining (red) at the plasma membrane, while GFP–PKD (green) was present within the cytosol of these cells (Figure 8, upper panels). When the GFP and CT signals were combined, no co-localization of GFP–PKD with the CT was observed (as indicated by the absence of yellow pseudocolour). Following activation of the BCR complex, the pattern of CT staining at the plasma membrane was altered, with numerous punctate spots of staining apparent (Figure 8, lower panels). These images also show the plasma membrane association of GFP–PKD following BCR ligation. When the confocal images were merged, remarkably little overlap (yellow) in the GFP–PKD (green) and the CT (red) staining at the plasma membrane was observed. These results indicate that GFP–PKD does not localize predominantly to lipid-enriched membrane microdomains in B lymphocytes following BCR engagement. It should be noted, however, that these experiments would not exclude the possibility that PKD might be localized transiently in the GEMs for a few seconds after BCR ligation, but this point could only be excluded by live imaging of cells expressing PKD and a co-expressed tagged marker for GEMs.
Fig. 8. GFP–PKD does not associate with lipid-rich membrane microdomains following BCR ligation. A20 B lymphocytes, transiently expressing GFP–PKD, were left untreated (n/s) or were treated with F(ab)′2 fragments of anti-mouse IgG for 2 min before the cells were fixed in 4% paraformaldehyde/PBS for 20 min at room tempertature. Lipid-rich membrane microdomains (GEMs) were visualized by staining for the microdomain-associated glycolipid GM1, using CT B subunit–biotin and streptavidin–Alexa568, as detailed in Materials and methods. CT staining is shown in red and GFP–PKD in green. Areas of co-localization are indicated in yellow pseudocolour. Results are representative of three independent experiments.
Discussion
The integration of spatial data with knowledge about protein activity is crucial for understanding signal transduction pathways. Herein, we used time-lapse confocal microscopy for real-time analysis of the spatial regulation of PKD during physiological conditions of lymphocyte activation and, importantly, integrated localization and catalytic activity data. Antigen receptor signalling initiates at the plasma membrane, but then must be transmitted into the cell interior and the nucleus. The present report identifies PKD as a signalling molecule that functions to amplify and disseminate antigen receptor-induced signals away from the plasma membrane. The intracellular localization of PKD is regulated dynamically by antigen receptors: the BCR and the FcεR1 induce the rapid activation and recruitment of PKD to the plasma membrane of B lymphocytes and mast cells. There are several striking features about this response. First, the total cellular pool of PKD translocates to the plasma membrane in response to antigen receptor ligation, indicating the strength of this response and identifying PKD as a point of signal amplification for antigen receptors. Secondly, PKD translocation to the membrane is transient: redistribution of PKD back to the cytosol of lymphocytes occurs ∼8–10 min after the initiation of antigen receptor stimulation, but strikingly is not accompanied by down-regulation of PKD catalytic activity. Rather, a sustained period of activation of PKD within the cytosol was observed, with high levels of enzyme activity seen hours after antigen receptor ligation. The fate of active PKD is in contrast to the fate of other DAG-regulated serine kinases such as classical/novel PKCs where plasma membrane translocation and activation are followed almost invariably by inactivation and/or targeting for proteolysis and down-regulation (Feng and Hannun, 1998; Oancea and Meyer, 1998; Hansra et al., 1999; Ng et al., 1999).
Previously, PKD was seen to localize preferentially to the Golgi in a hepatocellular carcinoma cell line and in human antral gastrin cells (Prestle et al., 1996; Moore et al., 1999). The present data show that PKD is not localized to the Golgi compartment in B lymphocytes or mast cells. Moreover, PKD is active at two different locations during lymphocyte activation. After antigen receptor engagement, PKD is active rapidly and transiently at the plasma membrane; however, during sustained antigen receptor activation, PKD is active and functions within the cytosol. The presence of PKD in the Golgi in some cells is intriguing and indicates that there may be cell-specific determinants controlling PKD subcellular distribution. It remains to be determined whether Golgi-localized PKD can ever be activated by physiological stimuli; invariably, studies with PKD (and many studies of PKCs) rely heavily on the use of pharmacological activators. In this context, the present study has compared spatial–temporal dynamics of PKD activity and localization in response to both physiological (antigen receptors) and pharmacological (phorbol esters) activators. The magnitude and duration of PKD activation by phorbol esters or physiological stimuli are identical, but in cells activated with phorbol esters, active PKD is continuously associated with the plasma membrane (or additionally with intracellular granule membranes in mast cells). In contrast, following antigen receptor ligation, active PKD dissociates from the plasma membrane and re-accumulates within the cytosol. It has been assumed previously that activation of DAG-regulated enzymes by phorbol esters or endogenous DAG differs both quantitatively and kinetically. Here we show that there are no quantitative or kinetic differences between phorbol esters and physiological stimuli in their ability to regulate PKD catalytic activity. There is, however, an unequivocal spatial difference in the localization of active PKD in response to physiological and pharmacological stimuli.
The present study has analysed the structural requirements for PKD localization in lymphocytes. Deletion of the PH domain of PKD results in the loss of the exclusive cytosolic location of the enzyme and allows the molecule to distribute between the nucleus and the cytosol. Nevertheless, PH domain-deleted PKD can still relocate to the plasma membrane in antigen receptor-activated cells, showing that other regions of the molecule control membrane translocation. The mechanism for uptake of the PH domain-deleted PKD into the nucleus is unknown, but this is an interesting result, which implies that the PKD PH domain has a structural role in controlling the localization of PKD in quiescent cells. The regulatory domain of PKD contains a DAG- and phorbol ester-binding CRD motif (Valverde et al., 1994; Van Lint et al., 1995; Dieterich et al., 1996). Here we have demonstrated that deletion or mutation of the PKD CRD has no apparent effect on the subcellular localization of the enzyme in quiescent cells, but completely abolishes antigen receptor-induced PKD translocation to the plasma membrane. These data clearly establish a role for the CRD as an essential structural determinant in regulating the rapid redistribution of PKD from the cytosol to the plasma membrane in antigen receptor-activated B lymphocytes and mast cells. The involvement of the DAG-binding CRD domain in PKD translocation to the membrane explains the transience of this process because lymphocytes express high levels of DAG kinases, which metabolize DAG to phosphatidic acid and rapidly eliminate this lipid following its production by PI(4,5)P2 hydrolysis. The actions of these DAG kinases in clearing DAG would ensure that the DAG-mediated association of PKD with the plasma membrane would be transient. Transient DAG elevation is almost invariably interpreted as transient activation of DAG-regulated kinases, and DAG-regulated serine kinases such as the PKCs are typically targeted for degradation after activation (Feng and Hannun, 1998; Oancea and Meyer, 1998). Interestingly, PKD activation is sustained once it leaves the membrane. The redistribution of active PKD to the cytosol is a novel process for a PKC superfamily member but the sustained activation of PKD away from the plasma membrane can be explained by the fact that PKD activity is dependent on its phosphorylation status and not on continued binding of DAG or other membrane phospholipids (Zugaza et al., 1996; Iglesias et al., 1998b).
Finally, antigen receptor signalling initiates in lipid microdomains or GEMs (Field, 1997; Stauffer, 1997; Xavier, 1998). PKD does not co-localize selectively with GEMs when at the plasma membrane, but seems to be excluded from these structures; this demonstrates how rapidly biochemical signals can propagate away from specialized membrane microdomains into the rest of the plasma membrane. Antigen receptor signalling has to be transmitted to the cell interior so that it can control the complex genetic changes that determine lymphocyte function. However, the signalling molecules that might be involved in this transmission process are not well defined. Herein we show that active PKD recycles from the plasma membrane back to the cytosol during the sustained response to antigen receptor ligation and is maintained in a catalytically active state for several hours. Hence, PKD looks like a molecule that transduces a transient signal generated by antigen receptors at the plasma membrane into a sustained signal in the cell interior.
Materials and methods
cDNA constructs
cDNA constructs containing various wild-type and mutant PKD sequences in the pcDNA3 mammalian expression vector have been described previously: PKD wild type; PKD ΔPH; PKD ΔCRD; PKD P287G and PKD D733A (Van Lint et al., 1995; Iglesias and Rozengurt, 1998; Iglesias et al., 1998a,b). Chimeric fusion proteins between GFP and different PKD mutants were generated by subcloning PKD constructs into the EcoRI site of a pEF-plink2-GFPC3 expression vector. All GFP constructs were verified by restriction enzyme analysis and sequencing. All constructs were purified by CsCl density gradient centrifugation before use in transient transfection experiments.
Cell culture and transient transfection
The murine B-lymphoma A20 cell line was maintained in RPMI-1640 medium, supplemented with 10% fetal bovine serum and 50 µM β-mercaptoethanol. The rat basophilic leukaemia cell line RBL 2H3 was maintained as described previously (Turner and Cantrell, 1997). For transient expression of PKD constructs, 1.5 × 107/0.5 µl A20 B cells or RBL 2H3 cells were electroporated with 10 µg of cDNA at 310 V and 960 µF, resuspended in 5 ml of complete medium and left overnight to recover before stimulation. For live cell imaging experiments, cells were plated onto 35 mm sterile glass dishes overnight.
Cell imaging and immunofluorescence staining
The antigen receptor on A20 cells was stimulated using 10 µg/ml rabbit anti-mouse F(ab)′2 fragments for different times as indicated. RBL 2H3 cells were primed for 1 h with 1 µg/ml IgE anti-DNP (di-nitrophenol) (Sigma Chemical Co., St Louis, MO), followed by antigenic cross-linking of the bound IgE using 500 ng/ml KLH–DNP (Calbiochem Corp.). For live cell imaging, cells were incubated in phenol red-free medium (supplemented with 20 mM HEPES) and placed inside a pre-warmed (37°C) chamber under a Zeiss Axiovert-100M inverted confocal microscope. GFP fluorescence was excited with an argon laser emitting at 488 nm and images were acquired using a 63×/NA 1.4 oil immersion objective and Zeiss LSM 510 software. Immunofluorescence staining for endogenous PKD or GM 130 was carried out by fixing cells in ice-cold methanol (–20°C) for 8 min before permeabilization with phosphate-buffered saline (PBS)/0.1% Triton X-100 at room temperature for 4 min. Sequential incubations in PBS/0.1% sodium borohydrate (10 min at room temperature) and PBS/0.2% gelatin (10 min at room temperature) were followed by incubation with polyclonal anti-PKD (sc-935; 1 µg/ml) or polyclonal GM 130 antiserum (ICRF; 1:200 dilution), as appropriate. The secondary layer was anti-rabbit Alexa488 or anti-rabbit Alexa568, respectively (Molecular Probes; 5 µg/ml). Both primary and secondary incubations were carried out at room temperature for 20 min. Staining of lipid rafts was carried out by incubating fixed cells with 5 µg/ml CT B subunit–biotin (Sigma) for 20 min at room temperature, followed by incubation with 5 µg/ml streptavidin–Alexa568 conjugate (Molecular Probes) for 20 min at room temperature. In all cases, the cells were washed in PBS after each stage and mounted in Gelvatol (Monsanto Chemicals), which was allowed to set for at least 4 h before confocal images were acquired, as described above.
Cell lysis and western blot analysis
Cells were lysed for 20 min at 4°C in 50 mM Tris–HCl pH 7.4, 2 mM EGTA, 2 mM EDTA, 1 mM dithiothreitol, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM 4-(2′-aminoethyl)-benzenesulfonyl fluoride hydrochloride and 1% Triton X-100. Lysates were clarified by centrifugation at 14 000 r.p.m. for 10 min at 4°C and proteins in the supernatant were acetone precipitated and resuspended in 2× SDS–PAGE sample buffer. Samples were resolved under reducing conditions by 8% SDS–PAGE and transferred to polyvinylidene difluoride membranes (Millipore). Western blot analysis was performed as indicated in the figure legends and immunoreactive bands were visualized by ECL.
In vitro kinase assays
PKD was immunoprecipitated from cell lysates at 4°C for 2 h with the PA-1 antiserum (1:100 dilution) described previously (Van Lint et al., 1995) and recovered with protein A–agarose beads. Immunocomplexes were washed twice in lysis buffer and once in kinase buffer (30 mM Tris–HCl pH 7.4, 10 mM MgCl2), and PKD activity (as measured by autophosphorylation) was determined by incubating immunocomplexes with 20 µl of kinase buffer containing 100 µM [γ-32P]ATP final concentration at 30°C for 10 min. Reactions were terminated by the addition of 2× SDS–PAGE sample buffer and analysed by SDS–PAGE and autoradiography.
Cell fractionation
Cytosolic and membrane fractions of A20 B lymphocytes were prepared according to the method of Kabouridis et al. (1997). Proteins within the resulting fractions were precipitated using ice-cold acetone and subsequently analysed by SDS–PAGE and western blotting.
Materials
ECL reagents and [γ-32P]ATP (370 MBq/ml) were from Amersham International (UK). PDBu was from Sigma. Protein A–agarose was from Boehringer Mannheim. A rabbit polyclonal PKD antibody directed against the C-terminus of PKD, used for immunoblotting and immunofluorescence studies, was from Santa Cruz (sc-935). All other reagents were from standard suppliers or as indicated in the text.
Acknowledgments
Acknowledgements
We thank P.Parker (Protein Phosphorylation Laboratory, ICRF) and members of the Lymphocyte Activation Laboratory for useful discussions. The polyclonal GM 130 antibody was kindly provided by the Cell Biology Laboratory (ICRF) and the NAGT1–GFP construct was a generous gift from D.Shima (Cell Biology Laboratory, ICRF). This work was supported by the ICRF. E.R. is supported by NIH grant DK 55003-01.
References
- Berry N. and Nishizuka,Y. (1990) Protein kinase C and T cell activation. Eur. J. Biochem., 189, 205–214. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R. (1999) Generic signals and specific outcomes: signalling through Ca2+, calcineurin and NF-AT. Cell, 96, 611–614. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R. and Clipstone,N.A. (1994) Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu. Rev. Biochem., 63, 1045–1083. [DOI] [PubMed] [Google Scholar]
- Dieterich S., Herget,T., Link,G., Bottinger,H., Pfizenmaier,K. and Johannes,F.J. (1996) In vitro activation and substrates of recombinant, baculovirus expressed human protein kinase C µ. FEBS Lett., 381, 183–187. [DOI] [PubMed] [Google Scholar]
- Feng X. and Hannun,Y.A. (1998) An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem., 273, 26870–26874. [DOI] [PubMed] [Google Scholar]
- Feng X., Zhang,J., Barak,L.S., Meyer,T., Caron,M.G. and Hannun,Y.A. (1998) Visualization of dynamic trafficking of a protein kinase C βII/green fluorescent protein conjugate reveals differences in G protein-coupled receptor activation and desensitization. J. Biol. Chem., 273, 10755–10762. [DOI] [PubMed] [Google Scholar]
- Field K.A., Holowka,D. and Baird,B. (1997) Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane microdomains. J. Biol. Chem., 272, 4276–4280. [DOI] [PubMed] [Google Scholar]
- Hansra G., Garcia-Paramio,P., Prevostel,C., Whelan,R.D., Bornancin,F. and Parker,P.J. (1999) Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem. J., 342, 337–344. [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Seki,N., Hattori,A., Kozuma,S. and Saito,T. (1999) PKCν, a new member of the protein kinase C family, composes a fourth subfamily with PKCµ. Biochim. Biophys. Acta, 1450, 99–106. [DOI] [PubMed] [Google Scholar]
- Hope H.R. and Pike,L.J. (1996) Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol. Biol. Cell, 7, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias T. and Rozengurt,E. (1998) Protein kinase D activation by mutations within its pleckstrin homology domain. J. Biol. Chem., 273, 410–416. [DOI] [PubMed] [Google Scholar]
- Iglesias T., Matthews,S. and Rozengurt,E. (1998a) Dissimilar phorbol ester binding properties of the individual cysteine-rich motifs of protein kinase D. FEBS Lett., 437, 19–23. [DOI] [PubMed] [Google Scholar]
- Iglesias T., Waldron,R.T. and Rozengurt,E. (1998b) Identification of in vivo phosphorylation sites required for protein kinase D activation. J. Biol. Chem., 273, 27662–27667. [DOI] [PubMed] [Google Scholar]
- Jamora C., Yamanouye,N., Van Lint,J., Vandenheede,J.R., Faulkner,D.J. and Malhotra,V. (1999) Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell, 98, 59–68. [DOI] [PubMed] [Google Scholar]
- Kabouridis P.S., Magee,A.L. and Ley,S.C. (1997) S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J., 16, 4983–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers S.J., Vanhaesebroeck,B. and Waterfield,M.D. (1999) Signalling through phosphoinositide 3-kinases: the lipids take center stage. Curr. Opin. Cell Biol., 11, 219–225. [DOI] [PubMed] [Google Scholar]
- Leitges M., Schmedt,C., Guinamard,R., Davoust,J., Schaal,S., Stabel,S. and Tarakhovsky,A. (1996) Immunodeficiency in protein kinase Cβ-deficient mice. Science, 273, 788–791. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A. (1999) Structural basis for high-affinity phosphoinositide binding by pleckstrin homology domains. Biochem. Soc. Trans., 27, 617–624. [DOI] [PubMed] [Google Scholar]
- Matthews S.A., Pettit,G.R. and Rozengurt,E. (1997) Bryostatin 1 induces biphasic activation of protein kinase D in intact cells. J. Biol. Chem., 272, 20245–20250. [DOI] [PubMed] [Google Scholar]
- Matthews S.A., Rozengurt,E. and Cantrell,D. (1999) Characterisation of S916 as an in vivo autophosphorylation site for PKD/PKCµ. J. Biol. Chem., 274, 26543–26549. [DOI] [PubMed] [Google Scholar]
- Mellor H. and Parker,P.J. (1998) The extended protein kinase C superfamily. Biochem. J., 332, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks C.R., Kupfer,H., Tamir,I., Barlow,A. and Kupfer,A. (1997) Selective modulation of protein kinase C-θ during T-cell activation. Nature, 385, 83–86. [DOI] [PubMed] [Google Scholar]
- Monks C.R., Freiberg,B.A., Kupfer,H., Sciaky,N. and Kupfer,A. (1998) Three-dimensional segregation of supramolecular activation clusters in T cells. Nature, 395, 82–86. [DOI] [PubMed] [Google Scholar]
- Montixi C., Langlet,C., Bernard,A.-M., Thimonier,J., Dubois,C., Wurbel,M.-A., Chauvin,J.-P., Pierres,M. and He,H.-T. (1998) Engagement of T cell receptor triggers its recruitment to low density detergent-insoluble microdomains. EMBO J., 17, 5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.D.W., Ring,M., Scriven,D.R.L., Smith,V.C., Meloche,R.M. and Buchan,A.M.J. (1999) The role of protein kinase C isozymes in bombesin-stimulated gastrin release from human antral gastrin cells. J. Biol. Chem., 274, 22493–22501. [DOI] [PubMed] [Google Scholar]
- Nakamura N.M., Rabouille,C., Watson,R., Nilsson,T., Hui,N., Slusarewicz,P., Kreis,T.E. and Warren,G. (1995) Characterisation of a cis-Golgi matrix protein, GM 130. J. Cell Biol., 131, 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A.C. (1997) Regulation of protein kinase C. Curr. Opin. Cell Biol., 9, 161–167. [DOI] [PubMed] [Google Scholar]
- Ng T. et al. (1999) Imaging protein kinase Cα activation in cells. Science, 283, 2085–2089. [DOI] [PubMed] [Google Scholar]
- Oancea E. and Meyer,T. (1998) Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell, 95, 307–318. [DOI] [PubMed] [Google Scholar]
- Oancea E., Teruel,M.N., Quest,A.F. and Meyer,T. (1998) Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signalling in living cells. J. Cell Biol., 140, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohamori S., Shirai,Y., Sakai,N., Fujii,M., Konishi,H., Kikkawa,U. and Saito,N. (1998) Three distinct mechanisms for translocation and activation of the δ subspecies of protein kinase C. Mol. Cell. Biol., 18, 5263–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher J.A., Touhara,K., Payne,E.S. and Lefkowitz,R.J. (1995) Pleckstrin homology domain-mediated membrane association and activation of the β-adrenergic receptor kinase requires coordinate interaction with G β γ subunits and lipid. J. Biol. Chem., 270, 11707–11710. [DOI] [PubMed] [Google Scholar]
- Prestle J., Pfizenmaier,K., Brenner,J. and Johannes,F.-J. (1996) Protein kinase Cµ is located at the Golgi compartment. J. Cell Biol., 134, 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Sasaki,K., Ikegaki,N., Shirai,Y., Ono,Y. and Saito,N. (1997) Direct visualisation of the translocation of the γ-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J. Cell Biol., 139, 1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets E.D., Holowka,D. and Baird,B. (1999) Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεR1 and their association with detergent-resistant membranes. J. Cell Biol., 145, 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima D.T., Haldar,K., Pepperkok,R., Watson,R. and Warren,G. (1997) Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol., 137, 1211–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko S.P., Law,C.L., Klaus,S.J., Chandran,K.A., Takata,M., Kurosaki,T. and Clark,E.A. (1996) Protein kinase Cµ (PKCµ) associates with the B cell antigen receptor complex and regulates lymphocyte signalling. Immunity, 5, 353–363. [DOI] [PubMed] [Google Scholar]
- Stauffer T.P. and Meyer,T. (1997) Compartmentalized IgE receptor-mediated signal transduction in living cells. J. Cell Biol., 139, 1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. (1998) Signalling through protein kinase C. Front. Biosci., 3, D1134–D1147. [DOI] [PubMed] [Google Scholar]
- Turner H. and Cantrell,D.A. (1997) Distinct Ras effector pathways are involved in Fc ε R1 regulation of the transcriptional activity of Elk-1 and NFAT in mast cells. J. Exp. Med., 185, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde A.M., Sinnett-Smith,J., Van Lint,J. and Rozengurt,E. (1994) Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl Acad. Sci. USA, 91, 8572–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint J., Sinnett-Smith,J. and Rozengurt,E. (1995) Expression and characterisation of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J. Biol. Chem., 270, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Van Lint J., Ni,Y., Valius,M., Merlevede,W. and Vandenheede,J.R. (1998) Platelet-derived growth factor stimulates protein kinase D through the activation of phospholipase Cγ and protein kinase C. J. Biol. Chem., 273, 7038–7043. [DOI] [PubMed] [Google Scholar]
- Xavier R., Brennan,T., Li,Q., McCormack,C. and Seed,B. (1998) Membrane compartmentation is required for efficient T cell activation. Immunity, 8, 723–732. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trible,R.P. and Samelson,L.E. (1998) LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity, 9, 239–246. [DOI] [PubMed] [Google Scholar]
- Zugaza J.L., Sinnett-Smith,J., Van Lint,J. and Rozengurt,E. (1996) Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J., 15, 6220–6230. [PMC free article] [PubMed] [Google Scholar]
- Zugaza J.L., Waldron,R.T., Sinnett-Smith,J. and Rozengurt,E. (1997) Bombesin, vasopressin, endothelin, bradykinin and platelet-derived growth factor rapidly activate protein kinase D through a protein kinase C-dependent signal transduction pathway. J. Biol. Chem., 272, 23952–23960. [DOI] [PubMed] [Google Scholar]