Abstract
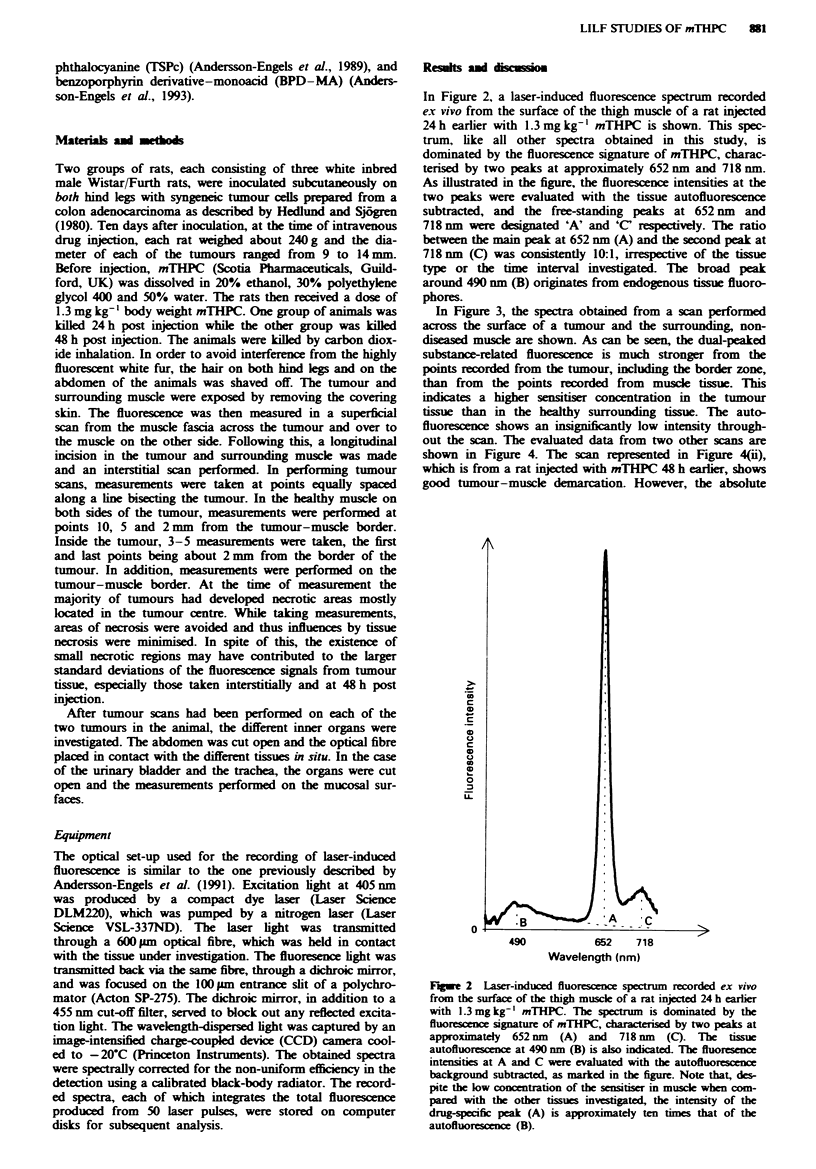
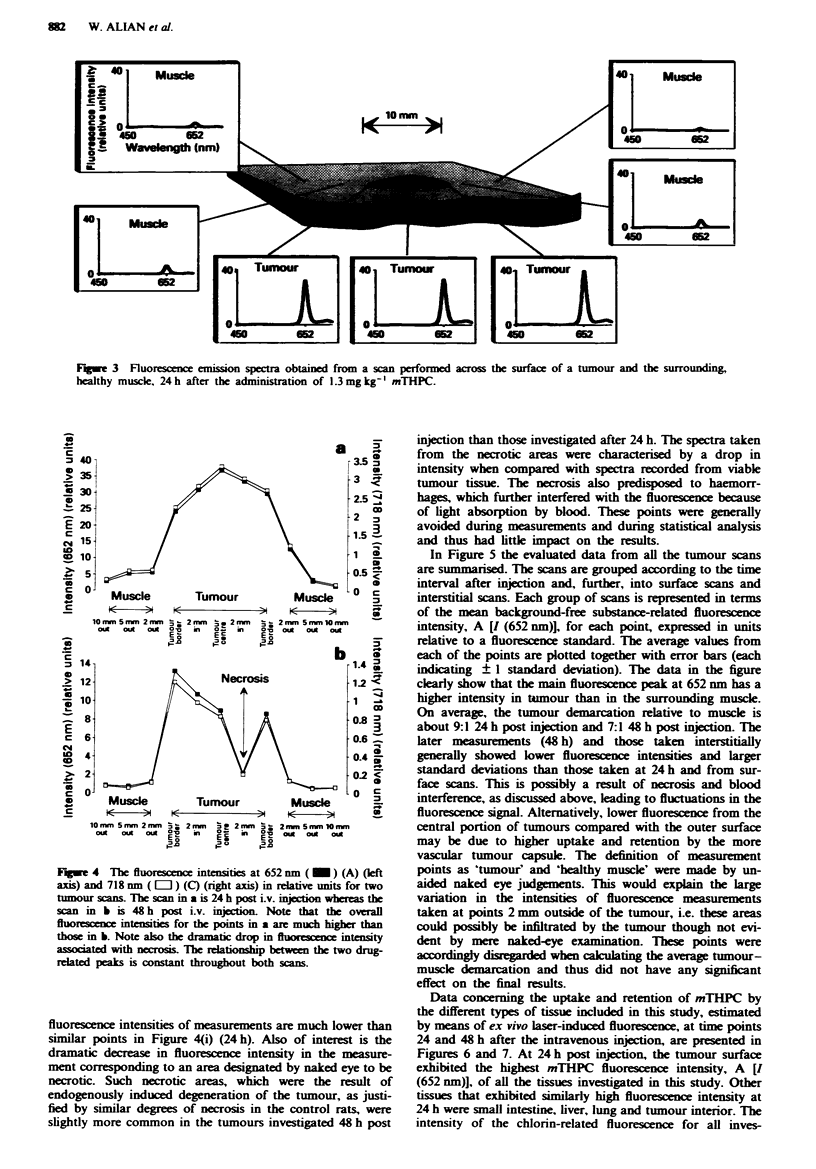
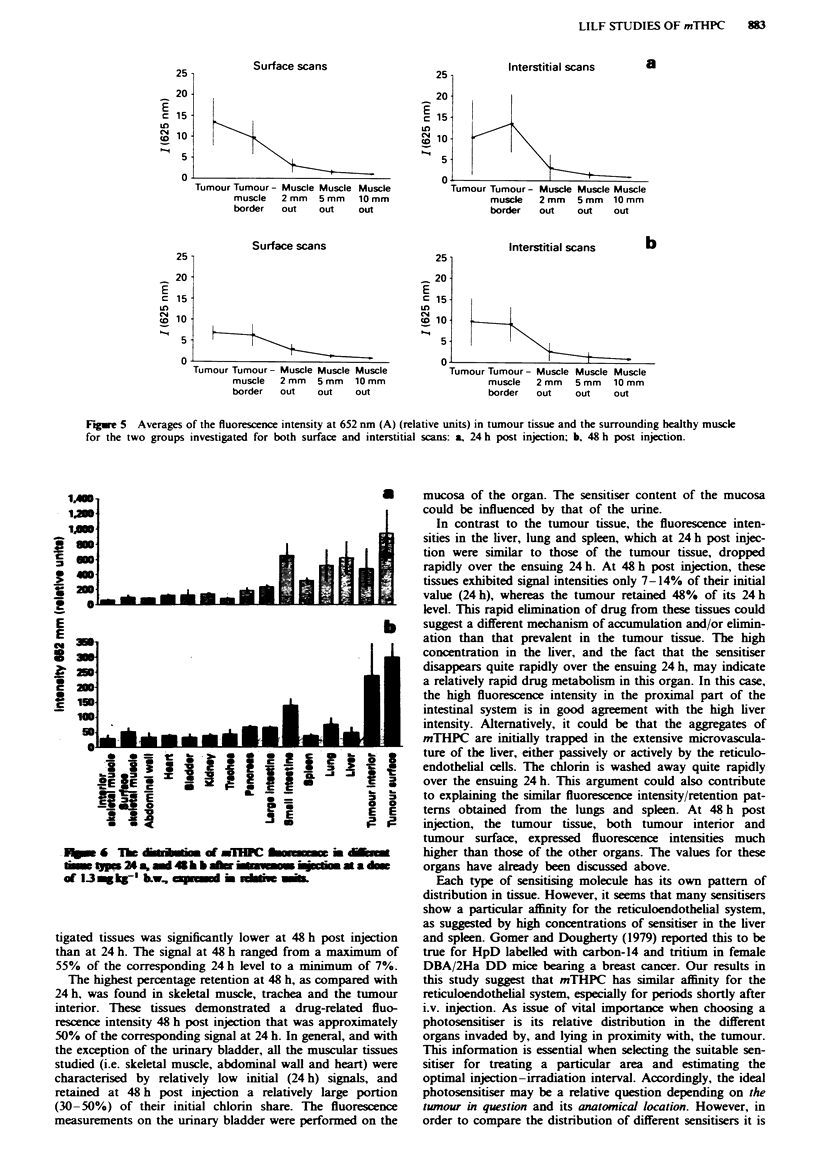
meso-Tetra(hydroxyphenyl)chlorin (mTHPC) is an attractive second-generation dihydroporphyrin photosensitiser for use in photodynamic therapy. In this study, 1.3 mg kg-1 body weight mTHPC was administered intravenously, and laser-induced fluorescence was used to characterise and compare its localisation and retention in different rat tissues, including an induced experimental adenocarcinoma, 24 h and 48 h post injection. These studies were performed in an attempt to predict the anatomical locations where mTHPC PDT might be most effective and suggest suitable injection--irradiation intervals in each case. Of particular interest were the intra-abdominal and intrathoracic tissues. The fluorescence was induced at 405 nm and the fluorescence spectrum in the region 450-750 nm was analysed. All collected spectra were dominated by the fluorescence signature of mTHPC with its peak at 652 nm, and all values in this study are in terms of background-free drug-specific fluorescence intensity at that wavelength. The photosensitiser accumulated in high concentrations in the tumour and the reticuloendothelial system. Muscular organs, such as the heart and the abdominal wall, were characterised by a low drug fluorescence signature.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson-Engels S., Ankerst J., Johansson J., Svanberg K., Svanberg S. Laser-induced fluorescence in malignant and normal tissue of rats injected with benzoporphyrin derivative. Photochem Photobiol. 1993 Jun;57(6):978–983. doi: 10.1111/j.1751-1097.1993.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Akande S. L., Bonnett R., Kaur H., Ioannou S., White R. D., Winfield U. J. meso-Tetra(hydroxyphenyl)porphyrins, a new class of potent tumour photosensitisers with favourable selectivity. Br J Cancer. 1986 Nov;54(5):717–725. doi: 10.1038/bjc.1986.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaney T. F., Sindelar W. F., Tochner Z., Smith P. D., Friauf W. S., Thomas G., Dachowski L., Cole J. W., Steinberg S. M., Glatstein E. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int J Radiat Oncol Biol Phys. 1993 Feb 15;25(3):445–457. doi: 10.1016/0360-3016(93)90066-5. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- Hedlund G., Sjögren H. O. Induction of transplantation immunity to rat colon carcinoma isografts by implantation of intact fetal colon tissue. Int J Cancer. 1980 Jul 15;26(1):71–73. doi: 10.1002/ijc.2910260111. [DOI] [PubMed] [Google Scholar]
- Moan J., Peng Q., Evensen J. F., Berg K., Western A., Rimington C. Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer. Photochem Photobiol. 1987 Nov;46(5):713–721. doi: 10.1111/j.1751-1097.1987.tb04837.x. [DOI] [PubMed] [Google Scholar]
- Nambisan R. N., Karakousis C. P., Holyoke E. D., Dougherty T. J. Intraoperative photodynamic therapy for retroperitoneal sarcomas. Cancer. 1988 Mar 15;61(6):1248–1252. doi: 10.1002/1097-0142(19880315)61:6<1248::aid-cncr2820610632>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pass H. I., Tochner Z., DeLaney T., Smith P., Friauf W., Glatstein E., Travis W. Intraoperative photodynamic therapy for malignant mesothelioma. Ann Thorac Surg. 1990 Oct;50(4):687–688. doi: 10.1016/0003-4975(90)90230-4. [DOI] [PubMed] [Google Scholar]
- Potter W. R., Mang T. S., Dougherty T. J. The theory of photodynamic therapy dosimetry: consequences of photo-destruction of sensitizer. Photochem Photobiol. 1987 Jul;46(1):97–101. doi: 10.1111/j.1751-1097.1987.tb04741.x. [DOI] [PubMed] [Google Scholar]
- Ris H. B., Altermatt H. J., Inderbitzi R., Hess R., Nachbur B., Stewart J. C., Wang Q., Lim C. K., Bonnett R., Berenbaum M. C. Photodynamic therapy with chlorins for diffuse malignant mesothelioma: initial clinical results. Br J Cancer. 1991 Dec;64(6):1116–1120. doi: 10.1038/bjc.1991.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H. B., Altermatt H. J., Nachbur B., Stewart J. C., Wang Q., Lim C. K., Bonnett R., Althaus U. Effect of drug-light interval on photodynamic therapy with meta-tetrahydroxyphenylchlorin in malignant mesothelioma. Int J Cancer. 1993 Jan 2;53(1):141–146. doi: 10.1002/ijc.2910530126. [DOI] [PubMed] [Google Scholar]
- Sindelar W. F., DeLaney T. F., Tochner Z., Thomas G. F., Dachoswki L. J., Smith P. D., Friauf W. S., Cole J. W., Glatstein E. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study. Arch Surg. 1991 Mar;126(3):318–324. doi: 10.1001/archsurg.1991.01410270062011. [DOI] [PubMed] [Google Scholar]
- Svanberg K., Kjellén E., Ankerst J., Montán S., Sjöholm E., Svanberg S. Fluorescence studies of hematoporphyrin derivative in normal and malignant rat tissue. Cancer Res. 1986 Aug;46(8):3803–3808. [PubMed] [Google Scholar]


