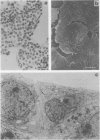
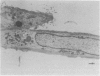
Abstract
Our previous studies showed that relaxin promotes differentiation of MCF-7 breast adenocarcinoma cells. In the current investigation, we aimed to elucidate whether the effect of the hormone is potentiated when MCF-7 cells are grown together with myoepithelial cells, thus creating a microenvironment reminiscent of the organised tissue architecture of the mammary parenchyma in vivo. The findings obtained reveal that most MCF-7 cells cultured alone have an undifferentiated, blast-like phenotype, only a minority showing a more differentiated phenotype with more organelles and rudimentary intercellular junctions. When co-cultured with myoepithelial cells more MCF-7 cells acquire ultrastructural features consistent with a more differentiated phenotype, such as a rich organellular complement, apical microvilli and intercellular junctions. When relaxin was added to the co-cultures, the ultrastructural signs of differentiation could be observed in even more MCF-7 cells and became more pronounced than in the absence of the hormone, judged by the appearance of a clear-cut polarisation of cytoplasmic organelles, an almost continuous coat of apical microvilli and numerous intracellular pseudolumina.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. The myoepithelium in human breast carcinoma. J Pathol. 1974 Jun;113(2):129–135. doi: 10.1002/path.1711130208. [DOI] [PubMed] [Google Scholar]
- Bani G., Bigazzi M., Bani D. Effects of relaxin on the mouse mammary gland. I. The myoepithelial cells. J Endocrinol Invest. 1985 Jun;8(3):207–215. doi: 10.1007/BF03348479. [DOI] [PubMed] [Google Scholar]
- Bani G., Bigazzi M., Bani D. The effects of relaxin on the mouse mammary gland. II. The epithelium. J Endocrinol Invest. 1986 Apr;9(2):145–152. doi: 10.1007/BF03348086. [DOI] [PubMed] [Google Scholar]
- Bigazzi M., Brandi M. L., Bani G., Sacchi T. B. Relaxin influences the growth of MCF-7 breast cancer cells. Mitogenic and antimitogenic action depends on peptide concentration. Cancer. 1992 Aug 1;70(3):639–643. doi: 10.1002/1097-0142(19920801)70:3<639::aid-cncr2820700316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gallo O., Bani D., Giudizi M. G., Biagiotti R., Almerigogna F., Toccafondi G., Fini-Storchi O., Romagnani S. Spontaneous in vitro differentiation of a myoepithelial cell line (PA 16/23) from a pleomorphic adenoma of the parotid gland is associated with reduced production of the autocrine growth factor interleukin 6. Br J Cancer. 1994 Jun;69(6):1065–1071. doi: 10.1038/bjc.1994.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo O., Bani D., Toccafondi G., Almerigogna F., Storchi O. F. Characterization of a novel cell line from pleomorphic adenoma of the parotid gland with myoepithelial phenotype and producing interleukin-6 as an autocrine growth factor. Cancer. 1992 Aug 1;70(3):559–568. doi: 10.1002/1097-0142(19920801)70:3<559::aid-cncr2820700304>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hwang J. J., Lee A. B., Fields P. A., Haab L. M., Mojonnier L. E., Sherwood O. D. Monoclonal antibodies specific for rat relaxin. V. Passive immunization with monoclonal antibodies throughout the second half of pregnancy disrupts development of the mammary apparatus and, hence, lactational performance in rats. Endocrinology. 1991 Dec;129(6):3034–3042. doi: 10.1210/endo-129-6-3034. [DOI] [PubMed] [Google Scholar]
- Kitajima K., Yamashita K., Fujita H. Fine structural aspects of follicle-like cavity formation from dispersed porcine thyroid cells cultured in a collagen substrate. Arch Histol Jpn. 1987 Mar;50(1):113–127. doi: 10.1679/aohc.50.113. [DOI] [PubMed] [Google Scholar]
- Mercado-Simmen R. C., Bryant-Greenwood G. D., Greenwood F. C. Relaxin receptor in the rat myometrium: regulation by estrogen and relaxin. Endocrinology. 1982 Jan;110(1):220–226. doi: 10.1210/endo-110-1-220. [DOI] [PubMed] [Google Scholar]
- Platica M., Chen H. Z., Ciurea D., Gil J., Mandeli J., Hollander V. P. Pituitary extract causes aggregation and differentiation of rat mammary tumor MTW9/Pl cells. Endocrinology. 1992 Dec;131(6):2573–2580. doi: 10.1210/endo.131.6.1446601. [DOI] [PubMed] [Google Scholar]
- Sacchi T. B., Bani D., Brandi M. L., Falchetti A., Bigazzi M. Relaxin influences growth, differentiation and cell-cell adhesion of human breast-cancer cells in culture. Int J Cancer. 1994 Apr 1;57(1):129–134. doi: 10.1002/ijc.2910570123. [DOI] [PubMed] [Google Scholar]
- Sakakura T. New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol. 1991;125:165–202. doi: 10.1016/s0074-7696(08)61219-x. [DOI] [PubMed] [Google Scholar]
- Sherwood C. D., O'Byrne E. M. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974 Jan;160(1):185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Vic P., Vignon F., Derocq D., Rochefort H. Effect of estradiol on the ultrastructure of the MCF7 human breast cancer cells in culture. Cancer Res. 1982 Feb;42(2):667–673. [PubMed] [Google Scholar]
- Yamashita K., Fujita H., Kitajima K., Nishii Y. Inter- and intracellular luminal formation in porcine thyroid tissues cultured in a collagen substrate. Arch Histol Cytol. 1989 May;52(2):109–114. doi: 10.1679/aohc.52.109. [DOI] [PubMed] [Google Scholar]
- Zou Z. Z., Petersen O. W., van Deurs B. Polarized expression of an apical membrane glycoprotein is established before functional tight junctions have developed in MCF-7 cells. J Histochem Cytochem. 1989 Jan;37(1):15–24. doi: 10.1177/37.1.2461981. [DOI] [PubMed] [Google Scholar]