Abstract
The POU domain transcription factor Oct-6 is a major regulator of Schwann cell differentiation and myelination. During nerve development and regeneration, expression of Oct-6 is under the control of axonal signals. Identification of the cis-acting elements necessary for Oct-6 gene regulation is an important step in deciphering the complex signalling between Schwann cells and axons governing myelination. Here we show that a fragment distal to the Oct-6 gene, containing two DNase I-hypersensitive sites, acts as the Oct-6 Schwann cell-specific enhancer (SCE). The SCE is sufficient to drive spatially and temporally correct expression, during both normal peripheral nerve development and regeneration. We further demonstrate that a tagged version of Oct-6, driven by the SCE, rescues the peripheral nerve phenotype of Oct-6-deficient mice. Thus, our isolation and characterization of the Oct-6 SCE provides the first description of a cis-acting genetic element that responds to converging signalling pathways to drive myelination in the peripheral nervous system.
Keywords: enhancer/myelination/POU factor/Schwann cell
Introduction
The development and proper function of peripheral nerves in vertebrates depend on the intimate interactions and continued signalling between Schwann cells and associated nerve fibre(s). In recent years, much progress has been made in identifying components of cell–cell interactions necessary in early stages of peripheral nerve development (Jessen and Mirsky, 1999). In contrast, very little is known about extracellular signals and intracellular signalling pathways that initiate myelination. Earlier work has indicated that the myelination programme of Schwann cells is under the control of the associated axon and correlates with axonal diameter (Aguayo et al., 1976a,b; Voyvodic, 1989). The nature of the axonal signal that drives myelination by Schwann cells remains unclear (Eldridge et al., 1989; Bunge et al., 1990). Whatever the exact nature of this signal, ultimately it must be relayed to the Schwann cell nucleus where transcription factors respond by co-ordinate regulation of sets of genes that mark and drive myelination.
Of particular interest are the transcription factors Krox-20 (Egr-2) and Oct-6 (SCIP or Tst-1), which are pivotal regulators of the transcriptional programme of myelination (Topilko et al., 1994; Bermingham et al., 1996; Jaegle et al., 1996). Neither of the two genes is expressed in the neural crest, the tissue from which the Schwann cells arise. However, within the sciatic nerve of the mouse, Schwann cells begin to express appreciable levels of Oct-6 from embryonic day 16 onwards (Blanchard et al., 1996; this study). Expression levels peak around birth, after which expression is extinguished gradually. Fully differentiated cells do not express the gene (Scherer et al., 1994; Zorick et al., 1996; Levavasseur et al., 1998). This down-regulation of Oct-6 gene expression is dependent on the Oct-6 gene product itself and on myelination per se (Jaegle and Meijer, 1998). Krox-20 expression is induced shortly after the first appearance of Oct-6 and becomes restricted to the myelinating lineage in which expression is maintained into adulthood (Zorick et al., 1996; Topilko et al., 1997). Expression of both genes is regulated by axonal contact (Monuki et al., 1990; Scherer et al., 1994; Zorick et al., 1996). Following nerve crush and Wallerian degeneration, when axonal contact is disrupted, reactive Schwann cells extinguish Krox-20 expression in denervated nerves. During nerve regeneration, axonal contact with Schwann cells is restored and Oct-6 expression is re-induced rapidly, and subsequently is followed by Krox-20 induction (Scherer et al., 1994; Zorick et al., 1996). In fully regenerated nerves, Oct-6 expression is down-regulated while Krox-20 expression remains high. Thus, both in development and regeneration, Schwann cell differentiation from early to late promyelinating to myelinating stages corresponds to the phenotypic progression from Oct-6+/Krox-20–, to Oct-6+/Krox-20+ to Oct-6–/Krox-20+.
Analysis of postnatal sciatic nerve development in Krox-20 and Oct-6 null mice indicates that both genes are required for normal progression of Schwann cell differentiation through the promyelin stage and initiation of the myelination programme. In both genetic mutants, Schwann cells appear morphologically arrested at the promyelin stage (Topilko et al., 1994; Bermingham et al., 1996; Jaegle et al., 1996). Oct-6 null Schwann cells eventually overcome this block and myelinate their associated axon normally, while Krox-20 null Schwann cells are blocked permanently at the promyelin stage. Induction of Oct-6 expression in Schwann cells does not require Krox-20 gene activity as Oct-6 is highly expressed in Krox-20 null Schwann cells (Zorick et al., 1999). Oct-6 and Krox-20 gene activity represent two subsequent steps in a genetic hierarchy that regulates progression of Schwann cell differentiation through the promyelin stage. Thus, Oct-6 is the first transcription factor in this cascade responding to axonal signalling. Hence, it is of importance to understand how this regulator itself is regulated.
As a first step in the systematic dissection of the regulation of Oct-6 gene expression during Schwann cell development, we set out to define the cis-acting genetic elements in the Oct-6 locus involved in the temporal and Schwann cell-specific expression of the gene. Through DNase I-hypersensitive site (HSS) mapping and deletional analysis in transgenic mice, we identified a fragment, located ∼12 kb downstream of the Oct-6 structural gene, which is sufficient to activate temporally correct expression in the Schwann cells of transgenic mice. Thus, Schwann cell specificity resides solely within this DNA fragment, which we named SCE for Oct-6 Schwann cell-specific enhancer. Furthermore, we show that the SCE/Oct-6 promoter-driven transgene is down-regulated in myelinating cells and re-induced in reactive Schwann cells of regenerating nerves following nerve crush. In addition, we show that the temporal delay in Schwann cell differentiation in Oct-6 null mice is rescued by a haemagglutinin (HA)-tagged Oct-6 gene expressed under the control of the SCE.
Results
A chromatin region spanning 45 kb of the Oct-6 locus contains eight DNase I-HSSs
The mouse Oct-6 gene is a single exon gene located on the distal arm of chromosome 4. The entire 3 kb long transcription unit is contained within a 7 kb EcoRI DNA fragment, and the promoter and 5′ part of the gene are located in a CpG island (Kuhn et al., 1991; Mandemakers et al., 1999). Previously, we have shown that the Oct-6 gene promoter is highly active in transient transfection assays irrespective of whether the transfected cell expresses endogenous Oct-6 or not (Mandemakers et al., 1999). In addition, this promoter does not drive specific expression of a reporter gene in transgenic mice. These preliminary experiments suggested that important regulatory elements are located outside the promoter region.
To begin a systematic dissection of the regulatory sequences within the Oct-6 locus, we first cloned and mapped an extensive region flanking the mouse Oct-6 gene (Figure 1A). Single copy DNA probes were isolated from this region and used to detect DNase I HSSs within chromatin of Oct-6-expressing versus non-expressing cells and tissues. HSSs are often found in the vicinity of promoter and enhancer regions of transcriptionally active genes and represent nucleosome-free sites in chromatin that are readily accessible to regulatory proteins (Gross and Garrard, 1988; Svaren and Chalkley, 1990; Felsenfeld et al., 1996). Thus, mapping HSSs within chromatin of the Oct-6 locus will reveal the position of DNA elements potentially involved in regulated Oct-6 gene expression.
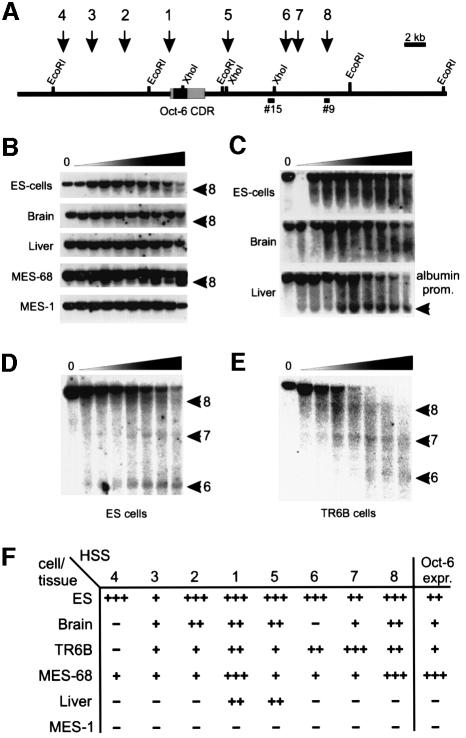
Fig. 1. DNase I HSS mapping in the mouse Oct-6 locus. (A) Map of the mouse Oct-6 locus. Several diagnostic restriction enzyme recognition sites are indicated. Black arrows and numbers above the locus indicate the positions of the corresponding HSSs. Bars below the map (#15 and #9) show the probes that were used for the HSS mappings presented in (B), (D) and (E). (B) Mapping of HSS8 in various cell lines and tissues. The band demonstrating the presence of HSS8 is indicated with a black arrow. This band is absent in untreated samples (0), but emerges with increasing intensity in samples treated with increasing amounts of DNase I (indicated by the triangle above the panels). HSS8 could only be detected in Oct-6-expressing cells. (C) The same samples for ES cells, brain and liver were used to verify whether any HSS could be detected in the liver series. Southern blots with EcoRI-digested DNA were hybridized with a probe derived from exon 1 of the albumin gene, to resolve the previously identified HSS coinciding with the mouse albumin promoter region (Liu et al., 1988). The band coinciding with this HSS was indeed only detected in the liver samples (black arrowhead). (D and E) The mapping of HSS6, HSS7 and HSS8 in ES cells and TR6B cells, respectively. Samples were digested with HpaI and hybridized with probe #15. These panels show that in ES cells, HSS6 and HSS8 are the most prominent sites, while in TR6B cells, HSS7 and HSS8 seem to be more sensitive than HSS6. (F) Relative degree of sensitivity of all HSSs in different cell lines and tissues.
We analysed Oct-6-expressing embryonic stem (ES) cells, MES-68 (an adenovirus E1 region transform P19 embryonic carcinoma cell derivative; Meijer et al., 1990), TR6B (a mouse Schwannoma cell line; Fields et al., 1975), adult mouse brain, and non-expressing mouse liver and the MES-1 (a P19 derivative) cell line. Nuclei from mouse cells were prepared and treated with DNase I. The presence of HSSs in chromatin is revealed by the appearance of discrete hybridizing bands on Southern blots with increasing DNase I concentration (Figure 1B–E). For example, HSS8 is detected with probe #9 in chromatin of MES-68, ES cells and brain, all of which express Oct-6, but not in non-expressing liver and MES-1 cells. The absence of HSS8 in chromatin of liver cells indeed correlates with the expression status of the Oct-6 gene in these cells and is not simply due to limited DNase I digestion as the albumin enhancer-associated HSS is readily detected in the same liver DNA samples (Figure 1C).
In total, we mapped eight HSSs within the Oct-6 locus (Figure 1A). The degree of DNase I sensitivity of these sites differs from cell line to cell line. For example, in ES cells, HSS6 is more pronounced than HSS7, while in TR6B cells HSS6 is less pronounced (also compare HSS8 sensitivity in ES cells and MES-68 cells in Figure 1B). The relative degree of sensitivity of the sites in different cell types is summarized in Figure 1F. The relatively low sensitivity of certain sites in brain most probably reflects the great diversity of cell types in this tissue, with only a small subset of cells expressing the Oct-6 gene.
A Schwann cell-specific enhancer resides in a DNA fragment containing two HSSs
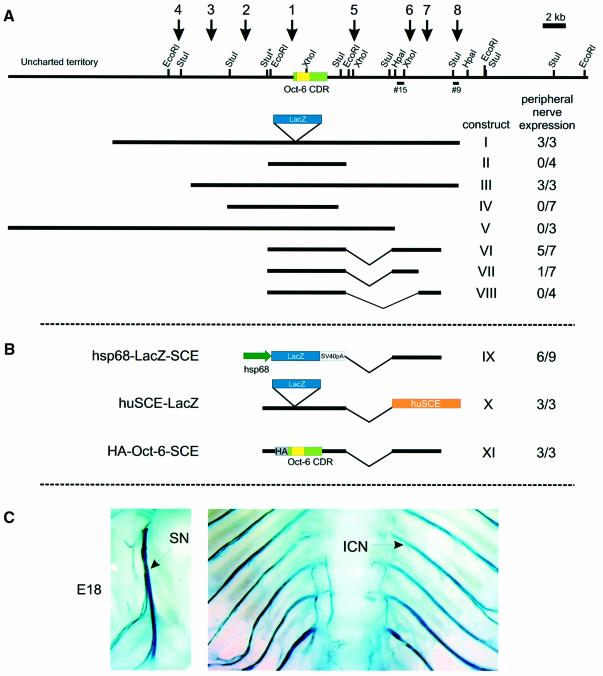
The relevance of these HSSs for Oct-6 gene regulation was tested in transgenic mice. The bacterial β-galactosidase gene was inserted in-frame in the Oct-6-coding region of a 32 kb genomic DNA construct containing all eight mapped HSSs (construct I, Figure 2). Three transgenic mouse lines were generated with this construct. Mice from all three lines expressed the β-galactosidase gene in the Schwann cells of their peripheral nerves. In addition, expression of LacZ was observed in many other tissues where Oct-6 is normally expressed, such as brain and skin, in particular the hair follicles. Next we generated transgenic mice with a smaller construct, lacking all HSSs except site 1 (construct II). These mice do not express the transgene in Schwann cells, although variable ectopic expression is seen in other tissues. These results indicate that the element necessary for Schwann cell-specific expression resides within the 32 kb region, but outside the 7 kb EcoRI fragment containing the Oct-6 structural gene.
Fig. 2. Deletion analysis. (A) Schematic representation of the mouse Oct-6 locus as described in Figure 1A, with the deletion constructs drawn to scale below. The E.coli β-galactosidase reporter gene (lacZ) was cloned in-frame into the Oct-6 coding region in every construct, as indicated for construct I. The number of transgenic animals displaying X-gal staining in the peripheral nerves out of the total number of transgenics obtained is shown to the right of each construct. (B) Schematic representation of the mouse SCE hsp68–LacZ construct (IX), the human SCE–LacZ construct (X) and the triple HA-tagged Oct-6 SCE construct (XI). (C) X-gal staining of peripheral nerves. Strong blue staining is observed at E18 in peripheral nerves of huSCE–LacZ (X) transgenic animals, as shown here, and in construct I, III, VI and IX transgenic animals (not shown). SN, sciatic nerve; ICN, intercostal nerve.
Schwann cell-specific expression was tested in founder animals, containing the other deletion constructs shown in Figure 2, at 18 days post-coitus (d.p.c.), a time at which Oct-6 is highly expressed in Schwann cells of the sciatic nerve. Construct III is 8 kb shorter than construct I at the 5′ end of the gene and lacks HSS4. This site was present in ES and MES-68 cells, but absent in the Schwannoma TR6B, brain and liver. All three transgenic founders obtained with construct III express LacZ in the Schwann cells of the sciatic nerve, indicating that HSS4 is dispensable for Schwann cell-specific expression. In addition, these three transgenics expressed LacZ in the hair follicles. Injections of construct IV, which contains only HSS2 and HSS1, yielded seven founder animals. None of these animals expressed the transgene in Schwann cells, but five showed variable expression in the brain and no expression in the hair follicles. Two transgenic lines and one founder, containing construct V, in which HSS6, 7 and 8 are missing, express LacZ in the hair follicles. However, neither of the construct V animals expressed the transgene in Schwann cells. These results pointed to the 3′ region encompassing HSS6, 7 and 8 as the region containing the DNA elements necessary for Schwann cell-specific expression. From this region, we isolated a 4.3 kb restriction fragment containing HSS6 and 7 and cloned it downstream of the Oct-6 structural gene in the Schwann cell non-expressing construct II. We generated five founders and two lines with construct VI. Four of the five founders and one of the two lines showed expression of the transgene in the sciatic nerve. Two of these founders expressed only in the nerves while the two other founders and the other line also showed brain and neural tube expression. None of the transgenic mice expressed the transgene in the hair follicles. In an attempt to narrow down this region further, we generated two constructs that contain either HSS6 (VII) or HSS7 (VIII). Four founders were obtained for construct VIII, none of which expressed in the Schwann cells of the sciatic nerve. Seven founders were obtained for construct VII, of which only one expressed in the sciatic nerve. In summary, these results show that a 4.3 kb fragment, containing HSS6 and HSS7 and located ∼12 kb downstream of the Oct-6 transcription initiation site, is sufficient to drive transgene expression from the Oct-6 promoter. We now refer to this fragment as the Oct-6 SCE.
The Oct-6 SCE confers Schwann cell specificity on the non-cell type-specific hsp68 promoter
Although the 4.3 kb SCE is sufficient for Schwann cell-specific expression of the transgene, it is possible that specific Oct-6 promoter-proximal elements contribute to the Schwann cell specificity of these transgenic constructs. To test this, we generated a construct in which the SCE is linked to the mouse minimal heat shock protein 68 (hsp68) gene promoter (construct IX in Figure 2B). This promoter contains a TATA-box, an SP1 site, a CCAAT box and three heat shock response elements downstream of the transcription initiation site. Others have shown that transgenic mice carrying hsp68 promoter-driven β- galactosidase transgenes show expression patterns that differ from line to line, the specific pattern depending on the site of integration (Kothary et al., 1989; Rossant et al., 1991). We generated three lines and six founders with construct IX. One of these lines and five founders express the transgene in the Schwann cell lineage, indicating that the SCE enhancer contains all the DNA elements to drive Schwann cell-specific expression from a basic promoter. Thus, it is unlikely that Oct-6 promoter-proximal elements contribute to full enhancer function of the SCE.
The SCE is conserved in humans
The Oct-6 gene is highly conserved among vertebrates and we have shown previously that the chicken and zebrafish Oct-6 genes are expressed in Schwann cells of both species (Levavasseur et al., 1998). We also showed that the dynamics of Oct-6 expression in the chick Schwann cell lineage are identical to those in mouse and rat, suggesting conservation of regulatory mechanisms as well. We took advantage of the relatively short evolutionary distance between human and mice to isolate, from a human cosmid library, clones that cover the 3′ part of the human Oct-6 gene. We generated an extensive restriction map of this region and isolated a 5 kb DNA fragment that cross-hybridizes to probe #15 and that we tentatively named human SCE (data not shown). This fragment was cloned in construct II, to generate construct X (Figure 2B) from which three transgenic lines were established. All three lines expressed the transgene at high levels in Schwann cells of the peripheral nerves (Figure 2C), indicating that the homologous human sequence has the same functional properties as the mouse SCE, corroborating the suggestion that Oct-6 regulatory mechanisms in the Schwann cell lineage are conserved.
The SCE is sufficient to drive temporally correct expression in the Schwann cell lineage
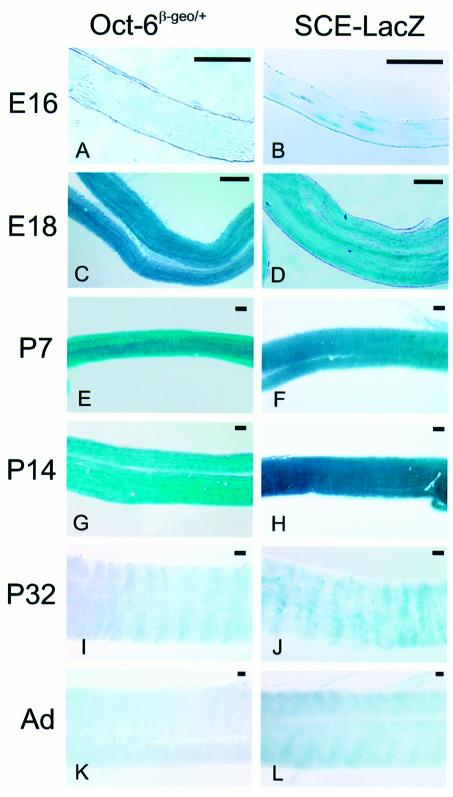
Thus far, our analysis has established that the Oct-6 SCE is sufficient to induce expression in the Schwann cell lineage. We then asked whether the SCE-directed expression is also temporally correct and, importantly, whether the SCE also mediates down-regulation of the reporter gene in cells that have exited the promyelin stage. Within the Schwann cell lineage of the mouse, Oct-6 protein expression can first be detected, using sensitive Oct-6 antibodies, in Schwann cell precursors that populate the sciatic nerve around embryonic day 12/13 (Blanchard et al., 1996). Oct-6 expression is strongly up-regulated at later embryonic stages, peaks during early postnatal stages and is extinguished in fully differentiated cells. Previously, we and others have described the inactivation of the mouse Oct-6 locus by insertion of a β-galactosidase–neomycin fusion gene (β-geo) (Bermingham et al., 1996; Jaegle et al., 1996). Expression of this fusion gene can be visualized easily in the developing embryo using the X-gal substrate, and recapitulates the Oct-6 expression profile as determined with Oct-6-specific antibodies and Oct-6 cRNA in situ hybridization (see Arroyo et al., 1998). As can be seen in Figure 3, essentially the same β-galactosidase expression profile is observed in nerves of transgenic mice carrying construct VI (the SCE–LacZ transgene) as in nerves of Oct-6β-geo/+ mice. Thus, the Oct-6 SCE does drive dynamic expression of a β-galactosidase transgene in the Schwann cell lineage in a temporal profile that is qualitatively identical to that of the endogenous Oct-6 gene. Importantly, the SCE–LacZ transgene is also down-regulated in mature nerves.
Fig. 3. Developmental expression of reporter gene activity in sciatic nerves of Oct-6β-geo/+ and SCE–LacZ mice. Sciatic nerves were isolated at various embryonic (E) and postnatal (P) stages, and stained for β-galactosidase activity. Whole-mount X-gal-stained sciatic nerves from Oct-6β-geo/+ animals are presented in (A), (C), (E), (G), (I) and (K). SCE–LacZ nerves are shown in (B), (D), (F), (H), (J) and (L). Low levels of expression could be detected at E16 in both mouse lines (A and B). High β-galactosidase expression is observed between E18 and P14 in both lines (C–H). At P32, few expressing cells could still be detected in the SCE–LacZ line (J), while X-gal staining was absent in the sciatic nerves of Oct-6β-geo/+ animals of the same age (I). LacZ expression could not be detected in nerves of adult animals from both lines (K and L). The scale bar for each panel is 100 µm.
Schwann cells express the SCE–LacZ transgene in response to cAMP in vitro
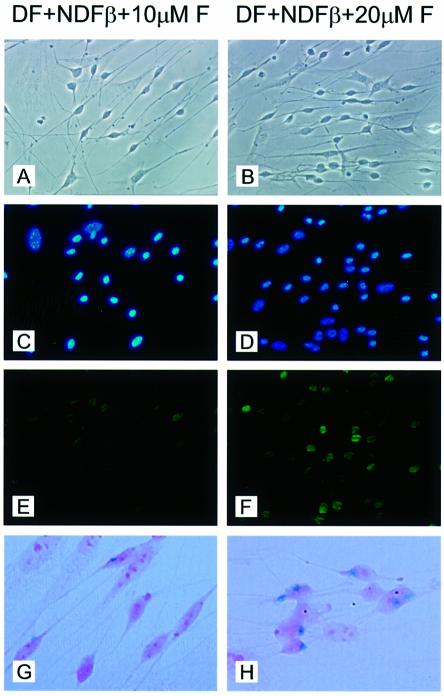
Oct-6 and myelin genes are detected together in actively myelinating Schwann cells of peripheral nerves during the first 2 weeks after birth. Disruption of axonal contact during this period, through either nerve transection or dissociation and culture of Schwann cells, results in a rapid down-regulation of Oct-6 and myelin gene expression (Scherer et al., 1994). Previously, it has been shown that elevation of intracellular levels of cAMP in cultured Schwann cells leads to a rapid induction of Oct-6 gene expression followed shortly afterwards by expression of myelin genes such as P0 and P2 (Monuki et al., 1989). These and related observations led to the suggestion that axonal signals operating during nerve development and regeneration are transduced, at least in part, through the adenylyl cyclase pathway. To test whether the cAMP signalling pathway could activate expression of the β-galactosidase transgene through the SCE, we established primary Schwann cell cultures from postnatal day 5 sciatic nerves from wild-type and SCE–LacZ transgenic mice. Cultures were exposed to different concentrations of forskolin, an activator of adenylyl cyclase. When Schwann cells are brought into culture, they rapidly down-regulate expression of the endogenous Oct-6 gene and the transgene. However, when cultured for 48 h in defined medium supplemented with 5% neu differentiation factor-β (NDF-β) conditioned medium, endogenous Oct-6 gene expression is re-induced in a forskolin concentration-dependent manner (compare Figure 4E and F). Under these conditions, LacZ expression is induced similarly in transgenic Schwann cells (compare Figure 4G and H), suggesting that cAMP induction of Oct-6 gene expression in vitro is mediated through the SCE.
Fig. 4. Induction of β-galactosidase expression in SCE–LacZ transgenic Schwann cells parallels induction of Oct-6 protein expression in response to forskolin. Primary Schwann cell cultures were established from wild-type and SCE–LacZ transgenic animals at postnatal day 4. Cells were cultured in defined medium (DF) supplemented with 5% NDF-β conditioned medium and exposed to 10 µM forskolin (A, C, E and G) or 20 µM forskolin (B, D, F and H) for 48 h (see Materials and methods). (A and B) Phase contrast photographs of primary Schwann cell cultures derived from wild-type animals. (C and D) 4′,6-Diamidino-2-phenylindole (DAPI) staining of nuclei of the cells shown in (A) and (B), respectively. (E and F) Many Schwann cells express Oct-6 (green) when exposed to 20 µM forskolin (F), while only few Schwann cells express Oct-6 when exposed to 10 µM forskolin (E). Fibroblasts, which are easily recognized by their flattened morphology and large nucleus, never express Oct-6. (G and H) Bright-field images of X-gal-stained SCE–LacZ primary Schwann cells. In cultures exposed to 20 µM forskolin (H), many cells with a Schwann cell morphology show a blue precipitate, while only few such cells are observed in the culture exposed to 10 µM forskolin. Fibroblasts, which are recognized by their flattened morphology and their pronounced nucleoli, do not express the transgene. Cells were counterstained with eosin.
Induction of Oct-6 gene expression in reactive Schwann cells during nerve regeneration is mediated through the SCE
Following nerve damage, axons disintegrate distal to the lesion, resulting in loss of axon–Schwann cell contact and dedifferentiation of Schwann cells (Wallerian degeneration). Denervated myelinating Schwann cells cease expression of myelin-related genes and reactivate expression of genes typical of immature cells, such as the low affinity neurotrophin receptor p75 and GAP-43 (Scherer and Salzer, 1996). Upon regeneration, axonal contact is restored and regrowing axons are ensheathed and myelinated by Schwann cells whose differentiation recapitulates the events during normal development, including the transient re-expression of Oct-6 (Scherer et al., 1994; Zorick et al., 1996).
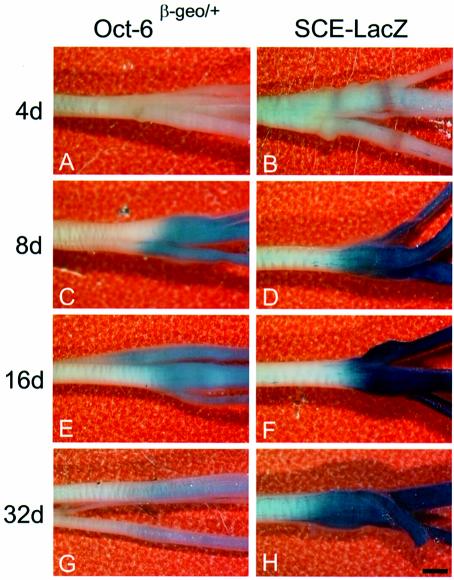
To test whether the SCE also mediates re-induction of Oct-6 expression in Schwann cells during regeneration, we performed X-gal staining of whole sciatic nerves at 4, 8, 16 and 32 days following crush lesion of the nerve in Oct-6β-geo/+ and SCE–LacZ transgenic mice (Figure 5). Re-activation of Oct-6 expression in Oct-6β-geo/+ nerves occurs between days 4 and 8 post-crush, and correlates with the re-ensheathment and myelination of regenerating axons. Expression of the Oct-6β-geo allele is then extinguished gradually and only low levels of expression are observed in nerves 32 days post-crush. In SCE–LacZ transgenic mice, reactivation of expression is seen during the same interval as in Oct-6β-geo/+ mice. Staining levels are most intense at 16 days post-crush, after which they begin to fade. The difference in staining intensity between the two different mouse lines probably reflects the difference in β-galactosidase copy numbers (one copy in Oct-6β-geo/+ and seven copies in SCE–LacZ mice). However, qualitatively, the SCE-driven transgene shows the same expression profile as the Oct-6β-geo/+ mice. Thus, the SCE also mediates reactivation of Oct-6 expression in Schwann cells during nerve regeneration. It is, therefore, likely that the same intracellular signalling pathway induces Oct-6 gene expression during development and during regeneration.
Fig. 5. Axonal regulation of reporter gene expression during nerve regeneration. Whole-mount X-gal staining was performed on crushed sciatic nerves of adult Oct-6β-geo/+ and SCE–LacZ animals at 4, 8, 16 and 32 days (d) after crush injury. (A and B) At 4 days after crush, no staining was visible in the nerve of Oct-6β-geo/+ animals, while some patchy staining could be observed distal to the site of crush in the SCE–LacZ nerve. (C and D) At 8 days after crush, strong staining can be detected in both mouse lines, but staining is more intense in the SCE–LacZ animal. (E and F) After 16 days, staining is already fading in the crushed Oct-6β-geo/+ sciatic nerve, while lacZ expression is still high in the SCE–LacZ nerve. (G and H) Only low levels of lacZ expression could be detected in the Oct-6 heterozygous animal 32 days after crush injury. Also, staining intensity in the SCE–LacZ nerve at 32 days has decreased compared with 16 days after crush (F). The scale bar is 1 mm and applies to all panels.
An SCE-driven HA-tagged Oct-6 transgene rescues the Oct-6 null peripheral nerve phenotype
Since the SCE is sufficient to mediate temporal regulation of Oct-6 gene expression within the Schwann cell lineage, it should be possible to rescue the peripheral nerve phenotype in Oct-6-deficient mice by expressing a tagged Oct-6 gene under the control of this enhancer element. To test this hypothesis, a DNA construct in which a triple HA-tagged Oct-6 gene is linked to the SCE (HA-Oct-6-SCE; construct XI in Figure 2) was used to generate three independent transgenic mouse lines, named HA-1, HA-2 and HA-3 and carrying 12, seven and three copies of the transgene, respectively. Using antibodies against the Oct-6 protein and the HA tag (data not shown), immunohistochemistry on sciatic nerves of newborn pups shows that all three lines expressed the transgene. These lines were crossed with Oct-6β-geo/+ mice to generate compound heterozygotes and offspring were backcrossed to Oct-6β-geo/+ mice. Viable HA/Oct-6β-geo/β-geo animals were born from each line. The number of surviving HA/Oct-6β-geo/β-geo animals was lower than expected (one-third of the expected number out of 200 live births), but higher than that of surviving Oct-6β-geo/β-geo animals (one-twentieth). It is possible that the higher survival rate of HA/Oct-6β-geo/β-geo animals is caused by a partial rescue of the breathing defect, which is thought to cause the high lethality in Oct-6β-geo/β-geo newborn animals (Bermingham et al., 1996). Apart from a slight reduction in size, HA/Oct-6β-geo/β-geo animals appeared normal.
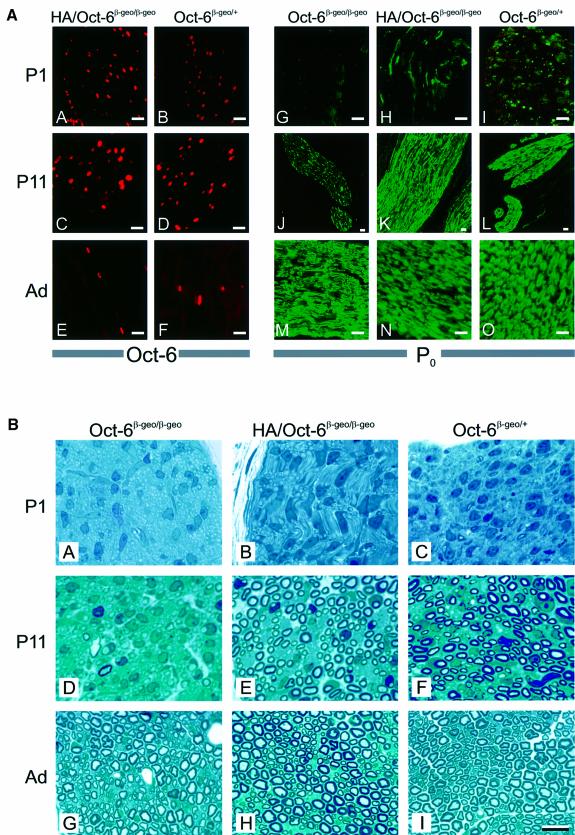
Pursuing our analysis with transgenic line HA-2, we observed that the expression profile of HA-Oct-6 in sciatic nerves of Oct-6β-geo/β-geo animals at P1, P11 and in adults (Figure 6A, panels A, C and E) was indistinguishable from the endogenous Oct-6 expression in Oct-6β-geo/+ animals (Figure 6A, panels B, D and F). From P1 to P11, many Schwann cells express the transgene in the Oct-6 null background, while in adult Oct-6 null mice the transgene is expressed in only a small number of cells.
Fig. 6. The SCE-driven HA-tagged Oct-6 transgene is expressed in Schwann cells and rescues the congenital hypomyelination phenotype in Oct-6β-geo/β-geo animals. (A) Developmental expression of HA-Oct-6 in the Schwann cells of Oct-6β-geo/β-geo animals (panels A, C and E) is qualitatively indistinguishable from that of endogenous Oct-6 in Oct-6β-geo/+ animals (panels B, D and F). Both the HA-tagged Oct-6 protein encoded by the transgene and the endogenous Oct-6 protein are detected here with an Oct-6-specific antibody (red fluorescence signal). P0 protein (green fluorescence signal) could not be detected in sciatic nerves of Oct-6β-geo/β-geo animals at P1 (panel G), while few Schwann cells express high levels of P0 protein in nerves of Oct-6β-geo/+ (H) and HA/Oct-6β-geo/β-geo (I) animals at this stage. At P11, high levels of P0 expression are seen in HA/Oct-6β-geo/β-geo and Oct-6β-geo/+ nerves (K and L), whereas in the Oct-6β-geo/β-geo only a small number of Schwann cells express high levels of the P0 protein (J). In adult sciatic nerve, high levels of P0 protein expression are observed in Schwann cells of all three genetic backgrounds, consistent with their myelination status (panels M, N and O; see also part B). The scale bar represents 10 µm. (B) Myelin appears on schedule in sciatic nerves of Oct-6β-geo/β-geo mice expressing the HA-Oct-6 transgene. Myelin is clearly visible as dark blue rings around axons in micrographs of nerves stained with methylene blue. At P1, no myelin could be detected in Oct-6β-geo/β-geo nerves (A), while few Schwann cells have elaborated a myelin sheath around axons in HA/Oct-6β-geo/β-geo and Oct-6β-geo/+ mice (B and C, respectively). At P11, myelin formation is well advanced in nerves of HA/Oct-6β-geo/β-geo and Oct-6β-geo/+ animals (E and F), while only a few myelin figures could be detected in nerves of Oct-6β-geo/β-geo animals. Although with considerable delay, myelination in Oct-6β-geo/β-geo animals does commence (Bermingham et al., 1996; Jaegle et al., 1996). The microscopic morphology of adult sciatic nerves of Oct-6β-geo/β-geo animals is nearly indistinguishable from that of adult HA/Oct-6β-geo/β-geo and Oct-6β-geo/+ sciatic nerves (H and I). The scale bar represents 10 µm and applies to all panels.
Previously, we have reported that the expression of one of the major structural myelin proteins, P0, is delayed in Schwann cells of Oct-6 null mice (Jaegle et al., 1996). We therefore examined whether the expression of P0 protein would be restored to normal in nerves from HA-2/Oct-6β-geo/β-geo mice. As is evident from Figure 6A (panels G–O), this is indeed the case, suggesting that the Schwann cells of HA-2/Oct-6β-geo/β-geo animals are actively myelinating. Also, light microscopic examination and comparison of semi-thin sections of sciatic nerves derived from HA-2/Oct-6β-geo/β-geo, Oct-6β-geo/β-geo and Oct-6β-geo/+ animals show that myelination is restored in Oct-6 null animals by SCE-driven HA-Oct-6 expression (Figure 6B). These data clearly demonstrate that the Oct-6 SCE is sufficient to mediate correct temporal expression at physiologically relevant levels of the Oct-6 gene during development as well as during nerve regeneration.
Discussion
Schwann cells are induced to adopt a one-to-one relationship with axons and initiate myelination under the influence of continued axonal signalling. The exact molecular nature of this signalling is not understood. However, one major target is the POU domain gene Oct-6. Oct-6 function is required in Schwann cells for normal progression of cell differentiation and myelination. We describe a cis-acting element, the SCE, within the Oct-6 locus on which intracellular signalling pathways converge to activate Oct-6 gene expression in response to this elusive axonal signal.
DNase I HSSs are associated with major regulatory elements in the Oct-6 locus
The mouse Oct-6 gene is expressed in a highly dynamic and complex pattern during embryonic development (Alvarez-Bolado et al., 1995; Zwart et al., 1996). Our preliminary experiments had shown that the gene promoter itself did not carry sufficient information to direct regulated expression in cell lines or transgenic mice (Mandemakers et al., 1999), suggesting that remote cis-acting elements regulate the Oct-6 expression pattern. As a first approach, we used DNase I hypersensitivity mapping to identify potential cis-acting elements in the Oct-6 locus. DNase I-hypersensitive regions in chromatin are often associated with important gene regulatory sequences such as enhancers, promoters and locus control regions (LCRs) (Gross and Garrard, 1988; Svaren and Chalkley, 1990; Felsenfeld et al., 1996). Eight such sites were mapped in the Oct-6 locus, six of which are only observed in Oct-6-expressing cells. The relevance of these HSSs for regulated Oct-6 expression in the Schwann cell lineage was assessed in transgenic mice using the bacterial β-galactosidase gene as a reporter. Differences in copy number between our transgenes (usually multicopy) and the one allele in the Oct-6β-geo/+ mice resulted in quantitative differences in β-galactosidase expression levels. Hence, β-galactosidase expression was used only as a qualitative indicator for temporo-spatial expression of Oct-6. We have made no attempt to correlate expression level (β-galactosidase activity) with copy number since most animals were analysed as founders and thus were potentially mosaic.
HSS1 is associated with the Oct-6 gene promoter, which consists of an atypical TATA-box (TTTAA) at position –23 and a GCCAAT-box at –80 relative to the transcriptional start site, and is embedded in a CpG island that extends well into the coding region of the gene. The presence of this site, together with HSS5, is not strictly correlated with Oct-6 gene expression. Both sites are also present in chromatin of cells that do not express Oct-6. The fact that this region is DNase I hypersensitive might reflect the unusual chromatin conformation associated with regions of high CG content (Antequera and Bird, 1999). Alternatively, it is possible that HSS1 is related to proliferation, as it has been suggested recently that CpG islands are associated with origins of DNA replication (Delgado et al., 1998; Antequera and Bird, 1999). However, HSS1 (and HSS5) is also present in chromatin of non-proliferating brain and liver cells. A third possibility is that HSS1 is part of a different unidentified transcription unit in the vicinity of the Oct-6 gene. Recently, we have identified such a transcription unit 3′ of the Oct-6 gene. This gene, which overlaps with HSS5, is expressed in heart, ES cells and testis, but not in liver or brainstem (our unpublished observations). Thus, the relationship between HSS1 and this new unidentified gene remains obscure.
The presence of HSS2 is correlated with Oct-6 expression in the panel of cell lines and tissues we examined. This site contains a previously identified oestrogen- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-responsive enhancer (Renner et al., 1996). Oestrogen and TPA were shown to enhance Oct-6 expression synergistically in oligodendroglia cell lines. Subsequent co-transfection in these cell lines indicated that the synergistic effect of TPA and oestrogen on Oct-6 expression was mediated through this enhancer. Earlier studies had shown that the oestrogen receptor is expressed in Schwann cells and oligodendrocytes and that oestrogens have an effect on Schwann cell proliferation and myelin gene expression (Jung-Testas and Baulieu, 1998). Together, these results led Renner et al. (1996) to suggest that this element mediates oestrogen regulation of Oct-6 gene expression and myelogenesis. However, our results demonstrate that this element is not required for regulated expression of the Oct-6 gene in the Schwann cell lineage in vivo.
HSS3 and HSS5 are both present in chromatin of the Schwannoma cell line TR6B, but are not sufficient or required to drive Schwann cell expression of the transgene that contains both these sites (construct V). This construct, together with constructs I and III, is, however, expressed in the hair follicles, another prominent site of Oct-6 expression (data not shown). It is, therefore, possible that either of the two sites, or both of them, is necessary for this aspect of the Oct-6 expression pattern.
Our analysis did not reveal any obvious function for HSS4 and HSS8. Both sites are dispensable for Schwann cell-specific expression. It is possible that these sites are involved in some other aspect of the Oct-6 expression pattern not analysed here.
Within the central nervous system (CNS), Oct-6 is expressed prominently in layers 2 and 5 of the cortex, the hippocampus and brainstem. None of the constructs that we tested was expressed consistently in these areas of the CNS (data not shown). Instead, transgene expression in the brain was highly variable from transgenic animal to animal, suggesting that these patterns result mainly from integration position effects. It is therefore likely that these aspects of Oct-6 expression are regulated by elements outside the region tested.
A 3 ′ distal enhancer in the Oct-6 locus mediates axonal regulation and Schwann cell-specific expression in a promoter-independent fashion
Schwann cell-specific expression was observed with most constructs that contained HSS6 and HSS7. A DNA restriction fragment containing these HSSs was termed the Oct-6 SCE and was shown further to be sufficient to drive temporally correct expression of a transgene, both during normal development and during nerve regeneration, and in vitro following stimulation by forskolin. Apparently, Oct-6 promoter-specific sequences are not required as the SCE also drives Schwann cell-specific expression from the hsp68 promoter. Furthermore, an HA-tagged version of the Oct-6 gene under the control of the SCE rescues the peripheral nerve phenotype observed in Oct-6 null animals. These results provide strong evidence that the Oct-6 SCE is sufficient to mediate all aspects of regulated Oct-6 gene expression in the Schwann cell lineage. Further deletion analysis of this fragment in which we separated HSS6 and HSS7 failed to give Schwann cell-specific expression, suggesting that both sites are necessary for full enhancer activity. In this respect, the Oct-6 SCE resembles other enhancers such as the 3′ enhancer of the stem cell leukaemia (SCL) gene, which also consists of two HSSs. Both sites are required for full SCL enhancer function in mast cells and early haematopoietic progenitors (Fordham et al., 1999; Sanchez et al., 1999). In fact, this is a rather common feature of many enhancers and LCRs. It reflects the modular nature of most of these elements in which bound nuclear factors need to interact to form a larger holo-complex to stimulate transcription from the linked promoter (Muller et al., 1988; Mannervik et al., 1999).
Despite our demonstration that the SCE is sufficient to drive all aspects of regulated Oct-6 expression in the Schwann cell lineage, not all transgenes containing the SCE are actually expressed in Schwann cells. We generated eight transgenic lines and 11 founders with various constructs that contained only HSS6 and HSS7, in addition to either the Oct-6 promoter or the hsp68 promoter. Of these 11 founders, nine expressed the transgene in the Schwann cell lineage. It is possible that the two mice that did not express this transgene were in fact highly chimeric, with no or only few transgenic cells contributing to the Schwann cell lineage. Chimerism, however, cannot explain why three out of the eight lines we generated do not express the transgene in Schwann cells. This is not due to a partial deletion of the transgene since Southern blotting confirmed the integrity of the transgene. It is most likely that these transgenes integrated in an unfavourable chromatin region that is not accessible in Schwann cells. Apparently, the SCE cannot overcome this negative integration position effect. In this context, it is of interest to note that all six transgenic lines generated with constructs I and III express in the Schwann cell lineage. These constructs contain, in addition to HSS6 and 7, several other HSSs (see Figure 2). It is possible that one of these sites contributes to the SCE overcoming negative position effects. Larger numbers of transgenic animals need to be generated and analysed to test this possibility. In addition, deletion of the SCE, through the route of homologous recombination in ES cells, will reveal whether HSS6 and HSS7 are required for Schwann cell-specific regulation or whether additional Schwann cell-specific elements are present in the Oct-6 locus.
The fact that Oct-6 gene expression in Schwann cells is under the control of axonal signals is perhaps illustrated most graphically during nerve regeneration when axonal contact with reactive Schwann cells is restored and Oct-6 gene expression is re-induced rapidly (Scherer et al., 1994). Thus, axonal signals are ultimately relayed to the Oct-6 SCE. This signalling does not involve the transcription factor Krox-20, as Krox-20 null promyelin-arrested Schwann cells express large amounts of Oct-6 (Zorick et al., 1999). It is, therefore, possible that Krox-20 is regulated in parallel with Oct-6 or that Krox-20 regulation depends on Oct-6. One possible intracellular signalling pathway involved in up-regulation of Oct-6 is the adenylyl cyclase–protein kinase A (PKA) pathway (Lemke and Chao, 1988; Monuki et al., 1989; Mirsky and Jessen, 1996). Elevation of cAMP, through activation of adenylyl cyclase with forskolin, in the presence of NDF-β leads to induction of Oct-6 expression in cultured Schwann cells and this induction is forskolin dose dependent (Figure 6). One major target of cAMP signalling is PKA, which in turn activates the transcription factor cAMP response element-binding protein (CREB) through phosphorylation on Ser133 (Gonzalez and Montminy, 1989). Also, NDF-β signalling has been shown to result in phosphorylation of CREB through a P21ras- and MAP kinase-dependent pathway (Kim et al., 1997; Tabernero et al., 1998). It is therefore possible that these pathways synergize in activation of CREB and stimulation of Oct-6 gene expression. As CREB is expressed in the Schwann cell lineage, this is a likely candidate nuclear factor to bind to the Oct-6 SCE. Indeed, potential CREB-binding sites are present in the SCE, while no such sites are found in the Oct-6 proximal promoter and this promoter is not active in Schwann cells of transgenic mice. The involvement of the cAMP pathway in this activation in vivo is, however, unclear as it has been shown that cAMP levels increase in the distal nerve stump only after activation of P0 expression (Poduslo et al., 1995). A critical assessment of the role of the PKA pathway in myelination will require the Schwann cell- and stage-specific inhibition of this pathway, for example by stage-specific expression of a dominant-negative form of the regulatory subunit of PKA.
Further detailed characterization of the Oct-6 SCE will identify transcription factors that bind to this element and on which intracellular signalling pathways converge to regulate Oct-6 gene expression and myelination. The identification of this unique enhancer does provide us with an important tool, not only to study those transcription factors and the signalling pathways that regulate their activity, but also to manipulate the expression of transgenes in Schwann cells during a defined period of their differentiation. It is, in particular, this last characteristic that makes this human SCE element very attractive in future gene therapy strategies for Schwann cells to be transplanted into lesioned nerves. Also, the demonstration here that an SCE-driven Oct-6 transgene rescues the peripheral nerve phenotype of Oct-6 null animals provides an experimental setting for the in vivo mapping of functional domains of the Oct-6 protein and to test possible Oct-6 redundant genes (Jaegle and Meijer, 1998).
Materials and methods
DNAse I HSS mapping and cloning
DNase I hypersensitvity mapping in chromatin was performed according to established methods with only minor modifications (Lichtsteiner et al., 1987). All DNA manipulations were carried out following described methods (Sambrook et al., 1989).
Transgenesis
DNA fragments were excised from constructs I–XI using appropriate restriction enzymes, separated on agarose gels, isolated by electroelution and purified using elutip-D-mini columns (Schleicher and Schuell). The DNA was dissolved in injection buffer (10 mM Tris–HCl pH 7.5, 0.08 mM EDTA pH 8.0) and introduced, by pronuclear injection, into fertilized eggs derived from an FVB/N × FVB/N mating as described (Hogan et al., 1994). Transgenic animals were analysed as founders or as lines. DNA samples of all animals were analysed by Southern blotting and hybridization to appropriate probes to confirm transgenesis. LacZ expression was visualized by whole-mount X-gal staining of E18 embryos or nerves dissected from transgenic animals at different stages of postnatal development.
Whole-mount X-gal staining
Embryos or dissected nerves (N. ischiaticus) were fixed for 30 min at room temperature in 2% formaldehyde (BDH), 0.2% glutaraldehyde (Sigma), 2 mM MgCl2, 5 mM EGTA pH 8.0, 0.02% NP-40 in phosphate-buffered saline (PBS) and processed further as described (Arroyo et al., 1998).
Primary Schwann cell cultures
Cultures of Schwann cells were set up as has been described before, with some modifications (Brockes et al., 1979; Kleitman et al., 1991). Dissected nerves were collected in L-15 Leibovitz. Nerves were transferred to L-15 medium containing 1 mg/ml collagenase (Roche), teased with dissection needles and incubated for 30 min at 37°C, with repeated pipetting to disrupt the nerves. Cells were washed once with L-15 containing 10% fetal calf serum (FCS), plated onto uncoated tissue culture dishes in Cb medium (Einheber et al., 1993), and incubated overnight at 37°C and 5% CO2. The next day, Schwann cells were harvested using the ‘Cold jet’ method (Jirsova et al., 1997), and plated onto collagen-coated coverslips. Cells were incubated for 48 h at 37°C and 5% CO2 in either Cb medium (containing 10% FCS) or in Defined medium (DF) (Murphy et al., 1996) supplemented with 5% NDF-β conditioned medium in the presence of 0, 10 or 20 µM forskolin (Sigma). A CHO cell line expressing a soluble form of NDF-β was cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12, 5% FCS for 7 days. Medium was collected and filter sterilized (NDF-β conditioned medium). Cells were fixed in a solution containing 35% acetone, 35% methanol, 5% acetic acid and 25% H2O for 10 min at room temperature and subsequently processed for immunochemistry or X-gal staining.
Sciatic nerve crush
Adult Oct-6β-geo/+ and SCE–LacZ mice were anaesthetized with halothane and the sciatic nerve was exposed. The sciatic nerve was crushed by tight compression with flattened Biology forceps no. 5 for 10 s at mid-femoral level just before the point where the sciatic nerve bifurcates. The mice were killed at 4, 8, 16 and 32 days after surgery, and their sciatic nerves were isolated and processed for whole-mount X-gal staining, with the unlesioned contralateral nerve serving as a control for background X-gal staining.
Immunohistochemistry
Sciatic nerves were isolated from mice derived from crosses between HA/Oct-6β-geo/+ and Oct-6β-geo/+ mice at the desired developmental stages, fixed overnight at 4°C in 35% acetone/35% methanol/5% acetic acid/25% H2O, dehydrated and paraffin embedded. All subsequent procedures have been described (Jaegle et al., 1996; Zwart et al., 1996). Primary antibodies used were: rabbit polyclonal anti-Oct-6 serum (Zwart et al., 1996), goat anti-HA serum (Santa Cruz Biotechnology), mouse monoclonal anti-P0 (Archelos et al., 1993) and mouse monoclonal anti-HA (12CA5; Roche). Fluorochrome-coupled secondary antibodies used were: goat anti-rabbit–Texas red (Molecular probes) and goat anti-mouse–Oregon green (Molecular probes). Both were used at 1:200 dilution.
Microscopy
Animals were perfused with PBS for 3 min, followed by fixative [3% paraformaldehyde (Sigma) and 1% glutaraldehyde buffered by 100 mM sodium cacodylate at pH 7.2] for 10 min. Sciatic nerves were isolated, washed with cacodylate buffer, osmicated in 1% osmium tetroxide and embedded in Epon. Semi-thin sections (1 µm) of Epon-embedded sciatic nerves were mounted on glass slides and stained with methylene blue.
Acknowledgments
Acknowledgements
The authors wish to thank Elaine Dzierzak and Sjaak Philipsen for their critical comments on the manuscript and Steve Scherer for stimulating discussions. Elior Peles and Yoshef Yarden are thanked for the NDF-β-expressing cell line and J.Archelos (Wurzburg) is thanked for monoclonal antibody against the P0 protein. Hans van den Berg’s assistance in animal surgery procedures is greatly acknowledged. This work was financed, in part, by grants from the Dutch Research Council (ALW) and the EC (Biomed 2 PL 962069).
References
- Aguayo A.J., Charron,L. and Bray,G.M. (1976a) Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J. Neurocytol., 5, 565–573. [DOI] [PubMed] [Google Scholar]
- Aguayo A.J., Epps,J., Charron,L. and Bray,G.M. (1976b) Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res., 104, 1–20. [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G., Rosenfeld,M.G. and Swanson,L.W. (1995) Model of forebrain regionalization based on spatiotemporal patterns of POU-III homeobox gene expression, birthdates and morphological features. J. Comp. Neurol., 355, 237–295. [DOI] [PubMed] [Google Scholar]
- Antequera F. and Bird,A. (1999) CpG islands as genomic footprints of promoters that are associated with replication origins. Curr. Biol., 9, R661–R667. [DOI] [PubMed] [Google Scholar]
- Archelos J.J., Roggenbuck,K., Schneider-Schaulies,J., Linington,C., Toyka,K.V. and Hartung,H.P. (1993) Production and characterization of monoclonal antibodies to the extracellular domain of P0. J. Neurosci. Res., 35, 46–53. [DOI] [PubMed] [Google Scholar]
- Arroyo E.J., Bermingham,J.R.,Jr, Rosenfeld,M.G. and Scherer,S.S. (1998) Promyelinating Schwann cells express Tst-1/SCIP/Oct-6. J. Neurosci., 18, 7891–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham J.R., Scherer,S.S., Oconnell,S., Arroyo,E., Kalla,K.A., Powell,F.L. and Rosenfeld,M.G. (1996) Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev., 10, 1751–1762. [DOI] [PubMed] [Google Scholar]
- Blanchard A.D., Sinanan,A., Parmantier,E., Zwart,R., Broos,L., Meijer,D., Meier,C., Jessen,K.R. and Mirsky,R. (1996) Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20 and Pax-3. J. Neurosci. Res., 46, 630–640. [DOI] [PubMed] [Google Scholar]
- Brockes J.P., Fields,K.L. and Raff,M.C. (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res., 165, 105–118. [DOI] [PubMed] [Google Scholar]
- Bunge M.B., Clark,M.B., Dean,A.C., Eldridge,C.F. and Bunge,R.P. (1990) Schwann cell function depends upon axonal signals and basal lamina components. Ann. N Y Acad. Sci., 580, 281–287. [DOI] [PubMed] [Google Scholar]
- Delgado S., Gomez,M., Bird,A. and Antequera,F. (1998) Initiation of DNA replication at CpG islands in mammalian chromosomes. EMBO J., 17, 2426–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S., Milner,T.A., Giancotti,F. and Salzer,J.L. (1993) Axonal regulation of Schwann cell integrin expression suggests a role for α 6 β 4 in myelination. J. Cell Biol., 123, 1223–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge C.F., Bunge,M.B. and Bunge,R.P. (1989) Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J. Neurosci., 9, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G., Boyes,J., Chung,J., Clark,D. and Studitsky,V. (1996) Chromatin structure and gene expression. Proc. Natl Acad. Sci. USA, 93, 9384–9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K.L., Gosling,C., Megson,M. and Stern,P.L. (1975) New cell surface antigens in rat defined by tumours of the nervous system. Proc. Natl Acad. Sci. USA, 72, 1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham J.L., Gottgens,B., McLaughlin,F. and Green,A.R. (1999) Chromatin structure and transcriptional regulation of the stem cell leukaemia (SCL) gene in mast cells. Leukemia, 13, 750–759. [DOI] [PubMed] [Google Scholar]
- Gonzalez G.A. and Montminy,M.R. (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell, 59, 675–680. [DOI] [PubMed] [Google Scholar]
- Gross D.S. and Garrard,W.T. (1988) Nuclease hypersensitive sites in chromatin. Annu. Rev. Biochem., 57, 159–197. [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lacy,L. (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Jaegle M. and Meijer,D. (1998) Role of Oct-6 in Schwann cell differentiation. Microsc. Res. Tech., 41, 372–378. [DOI] [PubMed] [Google Scholar]
- Jaegle M., Mandemakers,W., Broos,L., Zwart,R., Karis,A., Visser,P., Grosveld,F. and Meijer,D. (1996) The POU factor Oct-6 and Schwann cell differentiation. Science, 273, 507–510. [DOI] [PubMed] [Google Scholar]
- Jessen K.R. and Mirsky,R. (1999) Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci., 22, 402–410. [DOI] [PubMed] [Google Scholar]
- Jirsova K., Sodaar,P., Mandys,V. and Bar,P.R. (1997) Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J. Neurosci. Methods, 78, 133–137. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I. and Baulieu,E.E. (1998) Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J. Steroid Biochem. Mol. Biol., 65, 243–251. [DOI] [PubMed] [Google Scholar]
- Kim H.A., DeClue,J.E. and Ratner,N. (1997) cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J. Neurosci. Res., 49, 236–247. [PubMed] [Google Scholar]
- Kleitman N., Wood,P.M. and Bunge,R.P. (1991) Tissue culture methods for the study of myelination. In William,K. (ed.), Culturing Nerve Cells. Bradford Publishing Co., Acton, MA, pp. 337–377. [Google Scholar]
- Kothary R., Clapoff,S., Darling,S., Perry,M.D., Moran,L.A. and Rossant,J. (1989) Inducible expression of an hsp68–lacZ hybrid gene in transgenic mice. Development, 105, 707–714. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Monuki,E.S. and Lemke,G. (1991) The gene encoding the transcription factor SCIP has features of an expressed retroposon. Mol. Cell. Biol., 11, 4642–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. and Chao,M. (1988) Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development, 102, 499–504. [DOI] [PubMed] [Google Scholar]
- Levavasseur F., Mandemakers,W., Visser,P., Broos,L., Grosveld,F., Zivkovic,D. and Meijer,D. (1998) Comparison of sequence and function of the Oct-6 genes in zebrafish, chicken and mouse. Mech. Dev., 74, 89–98. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin,J. and Schibler,U. (1987) The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell, 51, 963–973. [DOI] [PubMed] [Google Scholar]
- Liu J.K., Bergman,Y. and Zaret,K.S. (1988) The mouse albumin promoter and a distal upstream site are simultaneously DNase I hypersensitive in liver chromatin and bind similar liver-abundant factors in vitro.Genes Dev., 2, 528–541. [DOI] [PubMed] [Google Scholar]
- Mandemakers W., Zwart,R., Kraay,R., Grosveld,G., Jaegle,A.G., Broos,L. and Meijer,D. (1999) Transcriptional regulation of the POU gene Oct-6 in Schwann cells. Adv. Exp. Med. Biol., 468, 13–22. [DOI] [PubMed] [Google Scholar]
- Mannervik M., Nibu,Y., Zhang,H. and Levine,M. (1999) Transcriptional coregulators in development. Science, 284, 606–609. [DOI] [PubMed] [Google Scholar]
- Meijer D., Graus,A., Kraay,R., Langeveld,A., Mulder,M.P. and Grosveld,G. (1990) The octamer binding factor Oct-6: cDNA cloning and expression in early embryonic cells. Nucleic Acids Res., 18, 7357–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R. and Jessen,K.R. (1996) Schwann cell development, differentiation and myelination. Curr. Opin. Neurobiol., 6, 89–96. [DOI] [PubMed] [Google Scholar]
- Monuki E.S., Weinmaster,G., Kuhn,R. and Lemke,G. (1989) SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron, 3, 783–793. [DOI] [PubMed] [Google Scholar]
- Monuki E.S., Kuhn,R., Weinmaster,G., Trapp,B.D. and Lemke,G. (1990) Expression and activity of the POU transcription factor SCIP. Science, 249, 1300–1303. [DOI] [PubMed] [Google Scholar]
- Muller M.M., Gerster,T. and Schaffner,W. (1988) Enhancer sequences and the regulation of gene transcription. Eur. J. Biochem., 176, 485–495. [DOI] [PubMed] [Google Scholar]
- Murphy P., Topilko,P., Schneider-Maunoury,S., Seitanidou,T., Baron-Van Evercooren,A. and Charnay,P. (1996) The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development, 122, 2847–2857. [DOI] [PubMed] [Google Scholar]
- Poduslo J.F., Walikonis,R.S., Domec,M.C., Berg,C.T. and Holtz-Heppelmann,C.J. (1995) The second messenger, cyclic AMP, is not sufficient for myelin gene induction in the peripheral nervous system. J. Neurochem., 65, 149–159. [DOI] [PubMed] [Google Scholar]
- Renner K., Sock,E., Bermingham,J.R.,Jr and Wegner,M. (1996) Expression of the gene for the POU domain transcription factor Tst-1/Oct6 is regulated by an estrogen-dependent enhancer. Nucleic Acids Res., 24, 4552–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Zirngibl,R., Cado,D., Shago,M. and Giguere,V. (1991) Expression of a retinoic acid response element–hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev., 5, 1333–1344. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sanchez M., Gottgens,B., Sinclair,A.M., Stanley,M., Begley,C.G., Hunter,S. and Green,A.R. (1999) An SCL 3′ enhancer targets developing endothelium together with embryonic and adult haematopoietic progenitors. Development, 126, 3891–3904. [DOI] [PubMed] [Google Scholar]
- Scherer S.S. and Salzer,J.L. (1996) Axon–Schwann cell interactions during peripheral nerve degeneration and regeneration. In Jessen,K.R. and Richardson,W.D. (eds), Glial Cell Development. BIOS Scientific Publishers Ltd, Oxford, UK, pp. 165–196. [Google Scholar]
- Scherer S.S., Wang,D.Y., Kuhn,R., Lemke,G., Wrabetz,L. and Kamholz,J. (1994) Axons regulate Schwann cell expression of the POU transcription factor SCIP. J. Neurosci., 14, 1930–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J. and Chalkley,R. (1990) The structure and assembly of active chromatin. Trends Genet., 6, 52–56. [DOI] [PubMed] [Google Scholar]
- Tabernero A., Stewart,H.J., Jessen,K.R. and Mirsky,R. (1998) The neuron–glia signal β neuregulin induces sustained CREB phosphorylation on Ser-133 in cultured rat Schwann cells. Mol. Cell. Neurosci., 10, 309–322. [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury,S., Levi,G., Baron-Van Evercooren,A., Chennoufi,A.B., Seitanidou,T., Babinet,C. and Charnay,P. (1994) Krox-20 controls myelination in the peripheral nervous system. Nature, 371, 796–799. [DOI] [PubMed] [Google Scholar]
- Topilko P., Levi,G., Merlo,G., Mantero,S., Desmarquet,C., Mancardi,G. and Charnay,P. (1997) Differential regulation of the zinc finger genes Krox-20 and Krox-24 (Egr-1) suggests antagonistic roles in Schwann cells. J. Neurosci. Res., 50, 702–712. [DOI] [PubMed] [Google Scholar]
- Voyvodic J.T. (1989) Target size regulates calibre and myelination of sympathetic axons. Nature, 342, 430–433. [DOI] [PubMed] [Google Scholar]
- Zorick T.S., Syroid,D.E., Arroyo,E., Scherer,S.S. and Lemke,G. (1996) The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol. Cell. Neurosci., 8, 129–145. [DOI] [PubMed] [Google Scholar]
- Zorick T.S., Syroid,D.E., Brown,A., Gridley,T. and Lemke,G. (1999) Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development, 126, 1397–1406. [DOI] [PubMed] [Google Scholar]
- Zwart R., Broos,L., Grosveld,G. and Meijer,D. (1996) The restricted expression pattern of the POU factor Oct-6 during early development of the mouse nervous system. Mech. Dev., 54, 185–194. [DOI] [PubMed] [Google Scholar]