Abstract
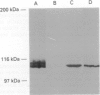
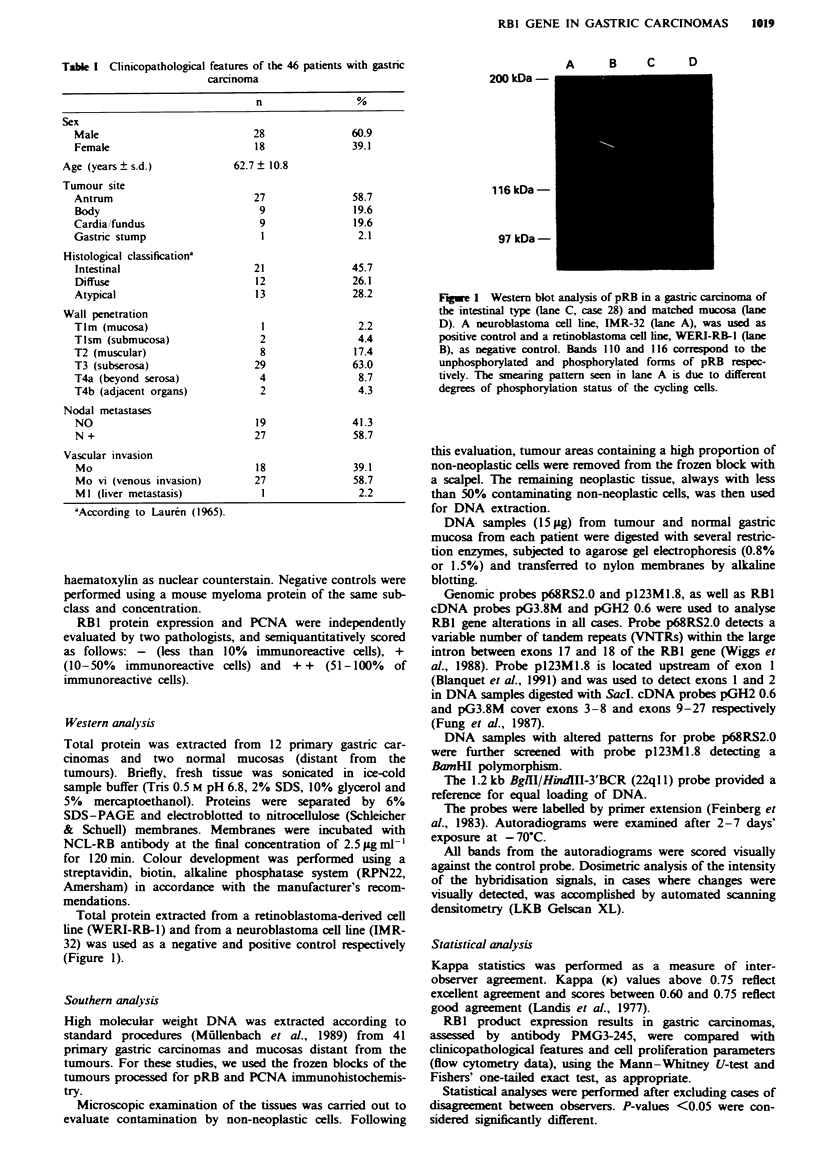
The role of the retinoblastoma gene (RB1) in human gastric carcinogenesis is yet to be clarified. We report on the analysis of RB1 structure and protein (pRB) expression in gastric carcinomas using Southern blotting, Western blotting and immunohistochemistry. The relationship between pRB expression and cell proliferation was assessed by a proliferation marker (PCNA) in a subset of cases. Non-neoplastic mucosas were studied, as controls, by the same methodology. We found a close relationship between pRB expression and PCNA in non-neoplastic mucosas as well as in gastric carcinomas. All tumours were immunohistochemically positive for pRB, although with a variable proportion of non-immunoreactive cells. Carcinomas of the diffuse type showed absence of pRB expression in a larger proportion of neoplastic cells than carcinomas of the intestinal type (P < 0.05). Analysis of the RB1 structure using probe p68RS2.0 revealed allelic imbalance in 29% of informative cases. No homozygous deletions and/or rearrangements were detected with p68RS2.0 and cDNA probes. Western analysis revealed no abnormal patterns of pRB. Our data therefore suggest that major alterations affecting the RB1 gene are rather infrequent in human gastric carcinomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanquet V., Turleau C., de Grouchy J., Creau-Goldberg N. Physical map around the retinoblastoma gene: possible genomic imprinting suggested by NruI digestion. Genomics. 1991 Jun;10(2):350–355. doi: 10.1016/0888-7543(91)90319-a. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Rio P., Madreperla S. A., Hong F., Allred C., Grizzle W. E., Lee W. H. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7762–7766. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg A., Zhang Q. X., Alm P., Olsson H., Sellberg G. The retinoblastoma gene in breast cancer: allele loss is not correlated with loss of gene protein expression. Cancer Res. 1992 May 15;52(10):2991–2994. [PubMed] [Google Scholar]
- Bártek J., Vojtesek B., Grand R. J., Gallimore P. H., Lane D. P. Cellular localization and T antigen binding of the retinoblastoma protein. Oncogene. 1992 Jan;7(1):101–108. [PubMed] [Google Scholar]
- Cairns P., Proctor A. J., Knowles M. A. Loss of heterozygosity at the RB locus is frequent and correlates with muscle invasion in bladder carcinoma. Oncogene. 1991 Dec;6(12):2305–2309. [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferti-Passantonopoulou A. D., Panani A. D., Vlachos J. D., Raptis S. A. Common cytogenetic findings in gastric cancer. Cancer Genet Cytogenet. 1987 Jan;24(1):63–73. doi: 10.1016/0165-4608(87)90083-5. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., DeCaprio J. A., Belvin M., Griffin J. D. Heterogeneous expression of the product of the retinoblastoma susceptibility gene in primary human leukemia cells. Oncogene. 1991 Aug;6(8):1343–1346. [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Ishikawa J., Xu H. J., Hu S. X., Yandell D. W., Maeda S., Kamidono S., Benedict W. F., Takahashi R. Inactivation of the retinoblastoma gene in human bladder and renal cell carcinomas. Cancer Res. 1991 Oct 15;51(20):5736–5743. [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Martínez J. C., Piris M. A., Sánchez-Beato M., Villuendas R., Orradre J. L., Algara P., Sánchez-Verde L., Martínez P. Retinoblastoma (Rb) gene product expression in lymphomas. Correlation with Ki67 growth fraction. J Pathol. 1993 Apr;169(4):405–412. doi: 10.1002/path.1711690404. [DOI] [PubMed] [Google Scholar]
- Motomura K., Nishisho I., Takai S., Tateishi H., Okazaki M., Yamamoto M., Miki T., Honjo T., Mori T. Loss of alleles at loci on chromosome 13 in human primary gastric cancers. Genomics. 1988 Feb;2(2):180–184. doi: 10.1016/0888-7543(88)90101-2. [DOI] [PubMed] [Google Scholar]
- Müllenbach R., Lagoda P. J., Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989 Dec;5(12):391–391. [PubMed] [Google Scholar]
- Ochi H., Douglass H. O., Jr, Sandberg A. A. Cytogenetic studies in primary gastric cancer. Cancer Genet Cytogenet. 1986 Aug;22(4):295–307. doi: 10.1016/0165-4608(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Reissmann P. T., Simon M. A., Lee W. H., Slamon D. J. Studies of the retinoblastoma gene in human sarcomas. Oncogene. 1989 Jul;4(7):839–843. [PubMed] [Google Scholar]
- Rosa J. C., Mendes R., Filipe M. I., Morris R. W. Measurement of cell proliferation in gastric carcinoma: comparative analysis of Ki-67 and proliferative cell nuclear antigen (PCNA). Histochem J. 1992 Feb;24(2):93–101. doi: 10.1007/BF01082445. [DOI] [PubMed] [Google Scholar]
- Saitoh K., Chiba T., Nakamura K. Cell proliferation kinetics of human gastric carcinoma: an immunohistochemical study with a monoclonal antibody against DNA polymerase-alpha. Eur J Cancer. 1992;28A(10):1642–1646. doi: 10.1016/0959-8049(92)90059-b. [DOI] [PubMed] [Google Scholar]
- Seruca R., Castedo S., Correia C., Gomes P., Carneiro F., Soares P., de Jong B., Sobrinho-Simões M. Cytogenetic findings in eleven gastric carcinomas. Cancer Genet Cytogenet. 1993 Jul 1;68(1):42–48. doi: 10.1016/0165-4608(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Shew J. Y., Ling N., Yang X. M., Fodstad O., Lee W. H. Antibodies detecting abnormalities of the retinoblastoma susceptibility gene product (pp110RB) in osteosarcomas and synovial sarcomas. Oncogene Res. 1989;4(3):205–214. [PubMed] [Google Scholar]
- T'Ang A., Wu K. J., Hashimoto T., Liu W. Y., Takahashi R., Shi X. H., Mihara K., Zhang F. H., Chen Y. Y., Du C. Genomic organization of the human retinoblastoma gene. Oncogene. 1989 Apr;4(4):401–407. [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Ikenaga M., Sugimoto M., Kotoura Y., Yamamuro T. Chromosomal reorganization for the expression of recessive mutation of retinoblastoma susceptibility gene in the development of osteosarcoma. Cancer Res. 1988 Jul 15;48(14):3939–3943. [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Wada M., Yokota J., Mizoguchi H., Sugimura T., Terada M. Infrequent loss of chromosomal heterozygosity in human stomach cancer. Cancer Res. 1988 Jun 1;48(11):2988–2992. [PubMed] [Google Scholar]
- Wiggs J., Nordenskjöld M., Yandell D., Rapaport J., Grondin V., Janson M., Werelius B., Petersen R., Craft A., Riedel K. Prediction of the risk of hereditary retinoblastoma, using DNA polymorphisms within the retinoblastoma gene. N Engl J Med. 1988 Jan 21;318(3):151–157. doi: 10.1056/NEJM198801213180305. [DOI] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Benedict W. F. Lack of nuclear RB protein staining in G0/middle G1 cells: correlation to changes in total RB protein level. Oncogene. 1991 Jul;6(7):1139–1146. [PubMed] [Google Scholar]
- Yokota J., Akiyama T., Fung Y. K., Benedict W. F., Namba Y., Hanaoka M., Wada M., Terasaki T., Shimosato Y., Sugimura T. Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene. 1988 Oct;3(4):471–475. [PubMed] [Google Scholar]