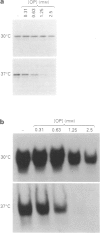
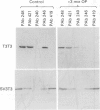
Abstract
The tumour-suppressor protein p53 is a metal-binding transcription factor with sequence-specific DNA-binding capacity. In cancer, mutation of p53 disrupts protein conformation with consequent loss of DNA binding and associated tumour-suppressor function. In vitro, the conformation and DNA-binding activity of wild-type p53 are subject to redox modulation and are abrogated by exposure to metal chelators. In the present study, we have used the chelator 1, 10-phenanthroline (OP) to probe the effect of temperature on the conformational stability of p53 translated in vitro. Whereas low temperature (30 degrees C) stabilised wild-type p53 conformation and protected against chelation, high temperature (41 degrees C) promoted destabilisation and enhanced chelation, indicating that temperature influences the folding of wild-type p53. Destabilisation of p53 tertiary structure induced protein aggregation through hydrophobic interactions, consistent with the notion that wild-type p53 contains a hydrophobic core which may become exposed by metal chelation. These results indicate that temperature sensitivity for conformation is an intrinsic property of wild-type p53 and suggests that small changes in temperature may directly affect p53 function.
Full text
PDF
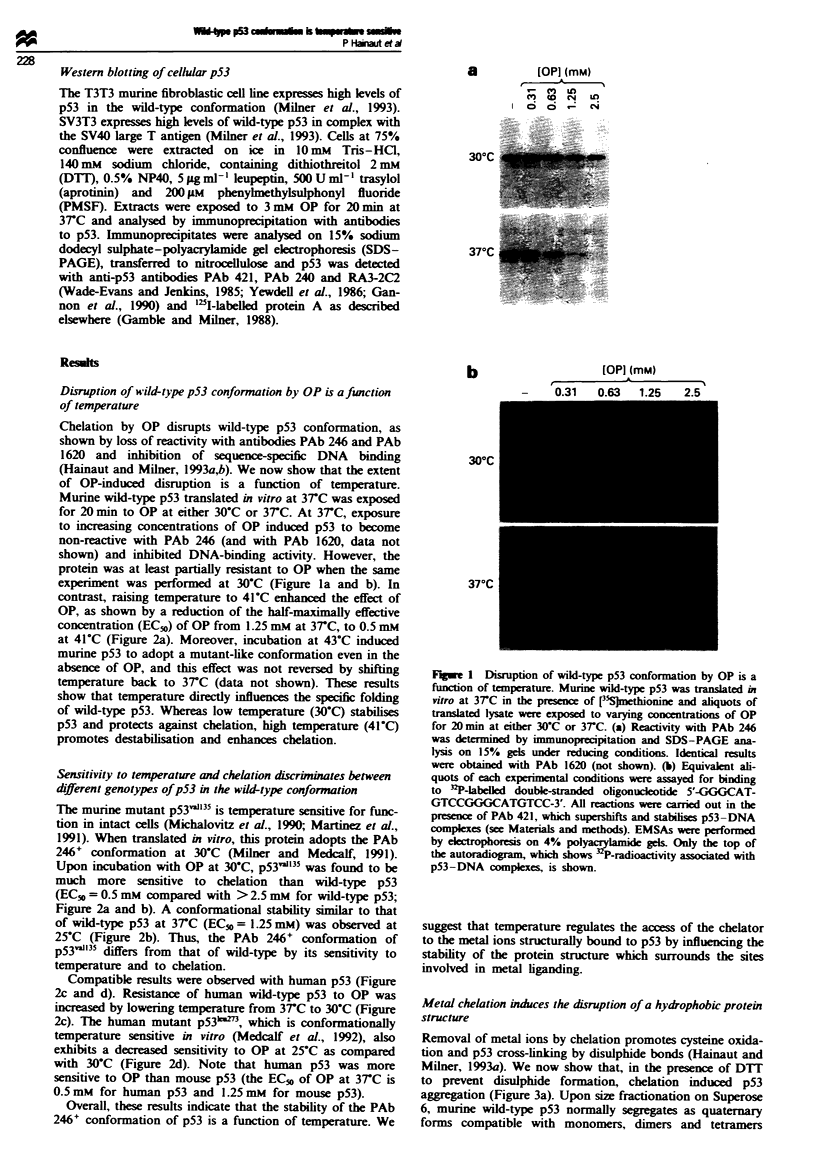
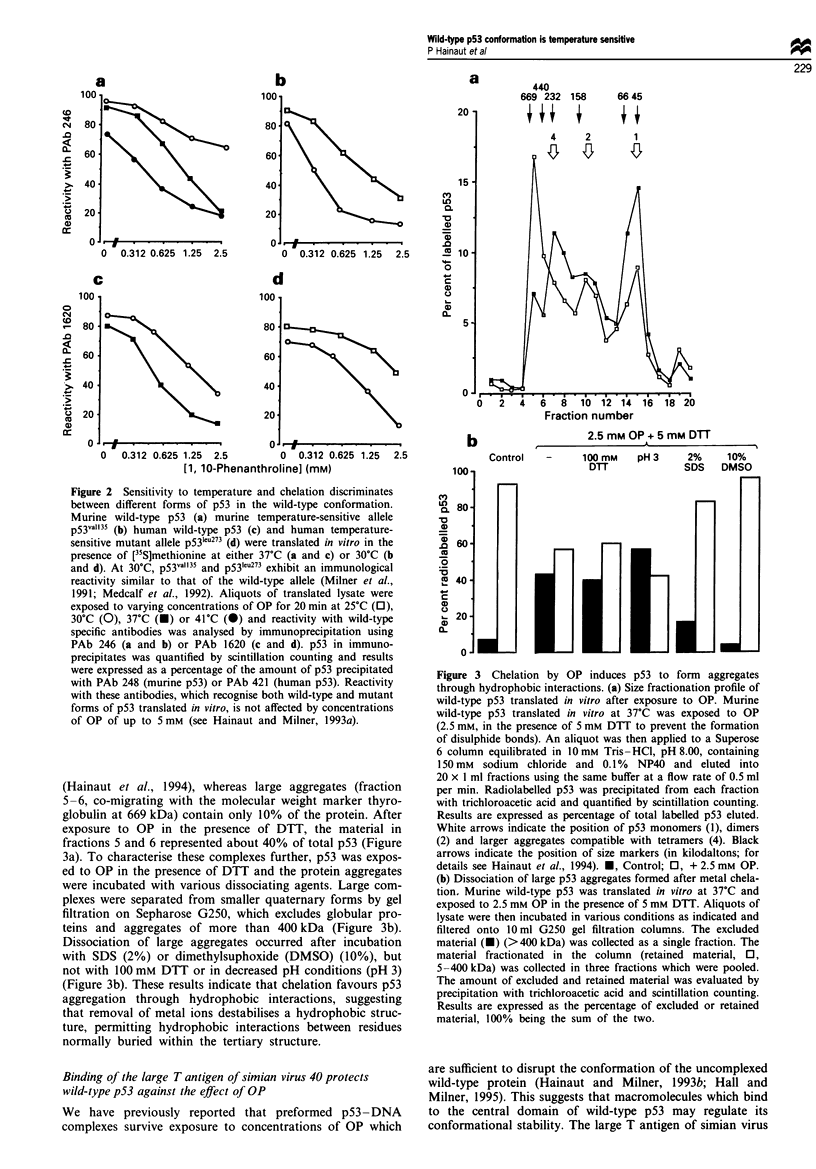

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour E. P., McEachern D., Wang Z., Corry P. M., Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993 Jun 15;53(12):2740–2744. [PubMed] [Google Scholar]
- Bargonetti J., Friedman P. N., Kern S. E., Vogelstein B., Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991 Jun 14;65(6):1083–1091. doi: 10.1016/0092-8674(91)90560-l. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Funk W. D., Wright W. E., Shay J. W., Minna J. D. Heterogeneity of transcriptional activity of mutant p53 proteins and p53 DNA target sequences. Oncogene. 1993 Aug;8(8):2159–2166. [PubMed] [Google Scholar]
- Cho Y., Gorina S., Jeffrey P. D., Pavletich N. P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994 Jul 15;265(5170):346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- Cook A., Milner J. Evidence for allosteric variants of wild-type p53, a tumour suppressor protein. Br J Cancer. 1990 Apr;61(4):548–552. doi: 10.1038/bjc.1990.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J., Milner J. Evidence that immunological variants of p53 represent alternative protein conformations. Virology. 1988 Feb;162(2):452–458. doi: 10.1016/0042-6822(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P., Hall A., Milner J. Analysis of p53 quaternary structure in relation to sequence-specific DNA binding. Oncogene. 1994 Jan;9(1):299–303. [PubMed] [Google Scholar]
- Hainaut P., Milner J. A structural role for metal ions in the "wild-type" conformation of the tumor suppressor protein p53. Cancer Res. 1993 Apr 15;53(8):1739–1742. [PubMed] [Google Scholar]
- Hainaut P., Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993 Oct 1;53(19):4469–4473. [PubMed] [Google Scholar]
- Halazonetis T. D., Kandil A. N. Conformational shifts propagate from the oligomerization domain of p53 to its tetrameric DNA binding domain and restore DNA binding to select p53 mutants. EMBO J. 1993 Dec 15;12(13):5057–5064. doi: 10.1002/j.1460-2075.1993.tb06199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Brill E., Shohat O., Prokocimer M., Wolf D., Arai N., Rotter V. Molecular basis for heterogeneity of the human p53 protein. Mol Cell Biol. 1986 Dec;6(12):4650–4656. doi: 10.1128/mcb.6.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Perry M. E., Chang A., Silver A., Dittmer D., Wu M., Welsh D. The 1993 Walter Hubert Lecture: the role of the p53 tumour-suppressor gene in tumorigenesis. Br J Cancer. 1994 Mar;69(3):409–416. doi: 10.1038/bjc.1994.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Mackey M. A., Anolik S. L., Roti Roti J. L. Cellular mechanisms associated with the lack of chronic thermotolerance expression in HeLa S3 cells. Cancer Res. 1992 Mar 1;52(5):1101–1106. [PubMed] [Google Scholar]
- Margulies L., Sehgal P. B. Modulation of the human interleukin-6 promoter (IL-6) and transcription factor C/EBP beta (NF-IL6) activity by p53 species. J Biol Chem. 1993 Jul 15;268(20):15096–15100. [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Medcalf E. A., Takahashi T., Chiba I., Minna J., Milner J. Temperature-sensitive mutants of p53 associated with human carcinoma of the lung. Oncogene. 1992 Jan;7(1):71–76. [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Milner J. A conformation hypothesis for the suppressor and promoter functions of p53 in cell growth control and in cancer. Proc Biol Sci. 1991 Aug 22;245(1313):139–145. doi: 10.1098/rspb.1991.0100. [DOI] [PubMed] [Google Scholar]
- Milner J., Chan Y. S., Medcalf E. A., Wang Y., Eckhart W. Partially transformed T3T3 cells express high levels of mutant p53 in the 'wild-type' immunoreactive form with defective oligomerization. Oncogene. 1993 Jul;8(7):2001–2008. [PubMed] [Google Scholar]
- Milner J., Medcalf E. A., Cook A. C. Tumor suppressor p53: analysis of wild-type and mutant p53 complexes. Mol Cell Biol. 1991 Jan;11(1):12–19. doi: 10.1128/mcb.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Temperature-dependent switching between "wild-type" and "mutant" forms of p53-Val135. J Mol Biol. 1990 Dec 5;216(3):481–484. doi: 10.1016/0022-2836(90)90371-R. [DOI] [PubMed] [Google Scholar]
- Milner J., Watson J. V. Addition of fresh medium induces cell cycle and conformation changes in p53, a tumour suppressor protein. Oncogene. 1990 Nov;5(11):1683–1690. [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Chambers K. A., Pabo C. O. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993 Dec;7(12B):2556–2564. doi: 10.1101/gad.7.12b.2556. [DOI] [PubMed] [Google Scholar]
- Ruppert J. M., Stillman B. Analysis of a protein-binding domain of p53. Mol Cell Biol. 1993 Jun;13(6):3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen C. W., Lane D. P. Mutant conformation of p53. Precise epitope mapping using a filamentous phage epitope library. J Mol Biol. 1992 Jun 5;225(3):577–583. doi: 10.1016/0022-2836(92)90386-x. [DOI] [PubMed] [Google Scholar]
- Wade-Evans A., Jenkins J. R. Precise epitope mapping of the murine transformation-associated protein, p53. EMBO J. 1985 Mar;4(3):699–706. doi: 10.1002/j.1460-2075.1985.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Guo X. Y., Hu G. Y., Liu W. B., Shay J. W., Deisseroth A. B. A temperature-sensitive mutant of human p53. EMBO J. 1994 Jun 1;13(11):2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]