Abstract
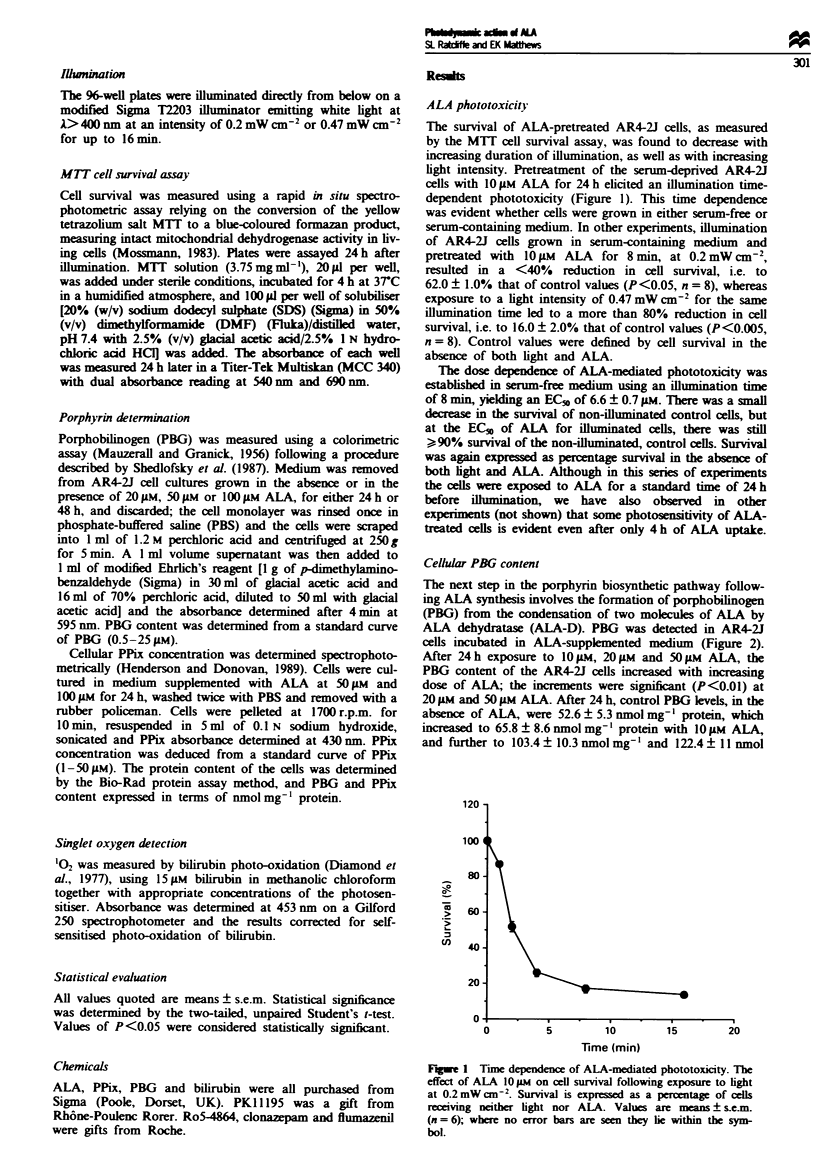
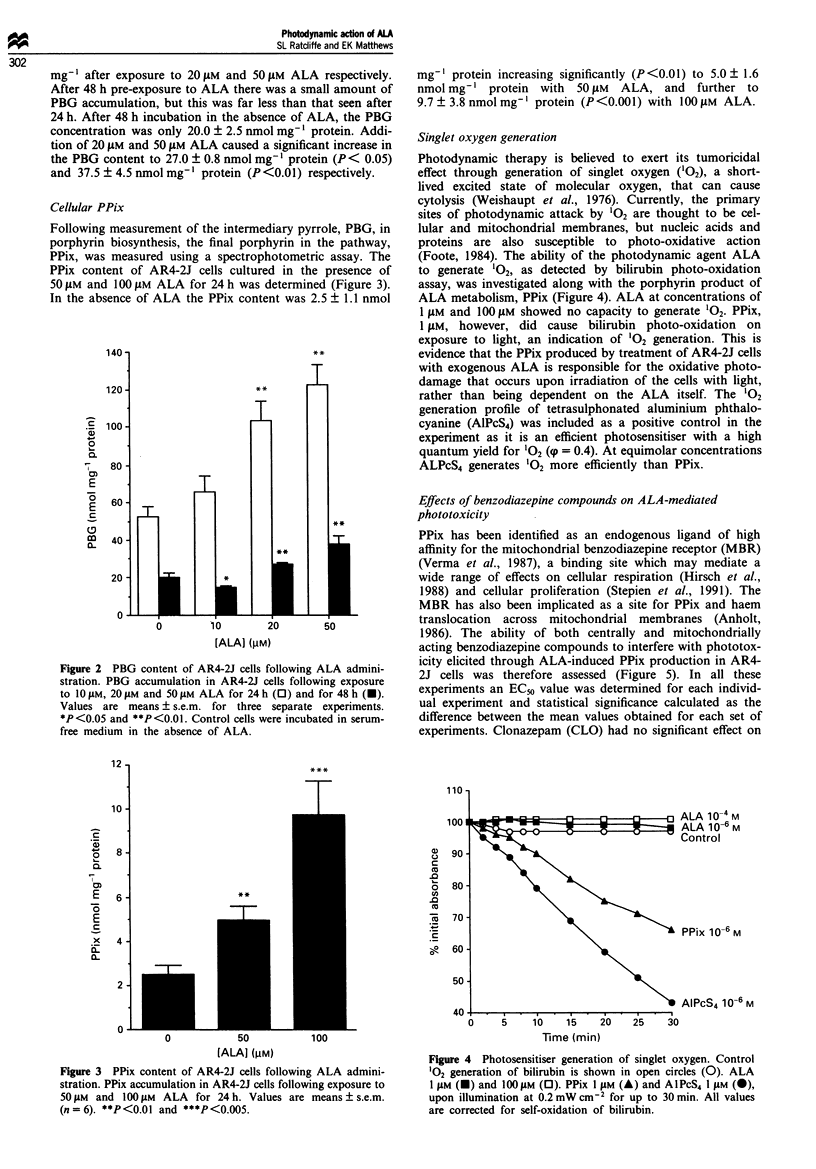
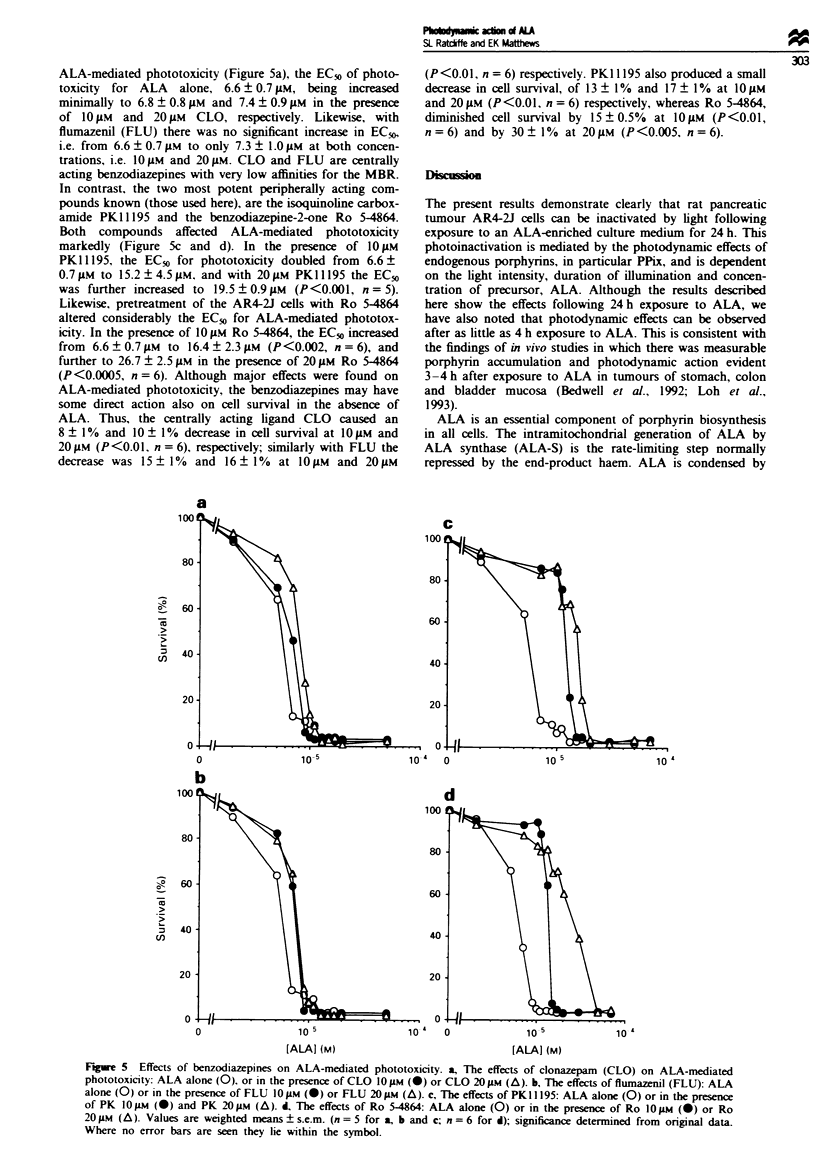
We have shown that addition of exogenous delta-aminolaevulinic acid (ALA) to rat pancreatoma AR4-2J cells in culture leads to the increased production of porphobilinogen (PBG) and the accumulation of photoactive protoporphyrin IX (PPix) in these cells. Exposure to light (lambda > 400 nm) at an intensity of 0.2 mW cm-2 for 8 min resulted in an ALA dose-dependent cytolysis of the cells, with an EC50 of 6.6 +/- 0.7 microM. This cytolytic effect was light intensity dependent, with greater cell destruction after exposure to light at an intensity of 0.47 mW cm-2 than at 0.2 mW cm-2; it was also dependent on the duration of illumination, cell survival decreasing with increasing illumination times. The photodestruction of the AR4-2J cells following exposure to ALA can be attributed to the production of endogenous PPix, a photoactive porphyrin that we have shown to generate singlet oxygen upon illumination, whereas ALA itself does not. Further investigation of the molecular mechanisms underlying the photodynamic action of ALA demonstrated the involvement of the mitochondrial (peripheral) benzodiazepine receptor (MBR), a high-affinity recognition site for dicarboxylic porphyrins, and especially PPix. The centrally acting benzodiazepine compounds clonazepam and flumazenil, which have negligible affinities for the MBR, had no effect on ALA-mediated phototoxicity. In contrast, both the isoquinoline carboxamide PK11195 and the benzodiazepine Ro 5-4864 ligands, displaying a high affinity for the MBR, did affect ALA-mediated phototoxicity, each markedly increasing the EC50 for cell photodestruction and thus exerting a photoprotective effect. It is concluded that the MBR may play an important role in the expression of ALA-mediated PPix phototoxicity and that MBR ligands, by diminishing the actions of endogenous PPix, have the potential to rescue cells from porphyrin-induced photolysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedwell J., MacRobert A. J., Phillips D., Bown S. G. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br J Cancer. 1992 Jun;65(6):818–824. doi: 10.1038/bjc.1992.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I., Granelli S. G., McDonagh A. F. Photochemotherapy and photodynamic toxicity: simple methods for identifying potentially active agents. Biochem Med. 1977 Apr;17(2):121–127. doi: 10.1016/0006-2944(77)90015-1. [DOI] [PubMed] [Google Scholar]
- Farges R., Joseph-Liauzun E., Shire D., Caput D., Le Fur G., Loison G., Ferrara P. Molecular basis for the different binding properties of benzodiazepines to human and bovine peripheral-type benzodiazepine receptors. FEBS Lett. 1993 Dec 13;335(3):305–308. doi: 10.1016/0014-5793(93)80407-l. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Donovan J. M. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 1989 Dec 15;49(24 Pt 1):6896–6900. [PubMed] [Google Scholar]
- Katz Y., Ben-Baruch G., Kloog Y., Menczer J., Gavish M. Increased density of peripheral benzodiazepine-binding sites in ovarian carcinomas as compared with benign ovarian tumours and normal ovaries. Clin Sci (Lond) 1990 Feb;78(2):155–158. doi: 10.1042/cs0780155. [DOI] [PubMed] [Google Scholar]
- Katz Y., Eitan A., Amiri Z., Gavish M. Dramatic increase in peripheral benzodiazepine binding sites in human colonic adenocarcinoma as compared to normal colon. Eur J Pharmacol. 1988 Apr 13;148(3):483–484. doi: 10.1016/0014-2999(88)90135-5. [DOI] [PubMed] [Google Scholar]
- Kessel D. Effects of photoactivated porphyrins at the cell surface of leukemia L1210 cells. Biochemistry. 1977 Jul 26;16(15):3443–3449. doi: 10.1021/bi00634a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Interactions between porphyrins and mitochondrial benzodiazepine receptors. Cancer Lett. 1988 Mar;39(2):193–198. doi: 10.1016/0304-3835(88)90104-8. [DOI] [PubMed] [Google Scholar]
- Loh C. S., MacRobert A. J., Bedwell J., Regula J., Krasner N., Bown S. G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer. 1993 Jul;68(1):41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker D. S., Lilja H. S., French J., Kuhlmann E., Noll W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979 Aug;7(4):197–202. doi: 10.1016/s0304-3835(79)80080-4. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Malik Z., Djaldetti M. Destruction of erythroleukemia, myelocytic leukemia and Burkitt lymphoma cells by photoactivated protoporphyrin. Int J Cancer. 1980 Oct 15;26(4):495–500. doi: 10.1002/ijc.2910260415. [DOI] [PubMed] [Google Scholar]
- Malik Z., Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer. 1987 Nov;56(5):589–595. doi: 10.1038/bjc.1987.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier R. H., Chow Y. F., LaPlante J. P., Truscott T. G., Kennedy J. C., Beiner L. A. Non-invasive technique for obtaining fluorescence excitation and emission spectra in vivo. Photochem Photobiol. 1986 Nov;44(5):679–687. doi: 10.1111/j.1751-1097.1986.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Rubino G. F., Rasetti L. Porphyrin metabolism in human neoplastic tissues. Panminerva Med. 1966 Jul-Aug;8(7):290–292. [PubMed] [Google Scholar]
- Sandberg S., Romslo I. Porphyrin-sensitized photodynamic damage of isolated rat liver mitochondria. Biochim Biophys Acta. 1980 Dec 3;593(2):187–195. doi: 10.1016/0005-2728(80)90056-0. [DOI] [PubMed] [Google Scholar]
- Shedlofsky S. I., Sinclair P. R., Bonkovsky H. L., Healey J. F., Swim A. T., Robinson J. M. Haem synthesis from exogenous 5-aminolaevulinate in cultured chick-embryo hepatocytes. Effects of inducers of cytochromes P-450. Biochem J. 1987 Nov 15;248(1):229–236. doi: 10.1042/bj2480229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien H., Kunert-Radek J., Stanisz A., Zerek-Melen G., Pawlikowski M. Inhibitory effect of porphyrins on the proliferation of mouse spleen lymphocytes in vitro. Biochem Biophys Res Commun. 1991 Jan 15;174(1):313–322. doi: 10.1016/0006-291x(91)90522-9. [DOI] [PubMed] [Google Scholar]
- Van Hillegersberg R., Van den Berg J. W., Kort W. J., Terpstra O. T., Wilson J. H. Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats. Gastroenterology. 1992 Aug;103(2):647–651. doi: 10.1016/0016-5085(92)90860-2. [DOI] [PubMed] [Google Scholar]
- Verma A., Nye J. S., Snyder S. H. Porphyrins are endogenous ligands for the mitochondrial (peripheral-type) benzodiazepine receptor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2256–2260. doi: 10.1073/pnas.84.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Snyder S. H. Characterization of porphyrin interactions with peripheral type benzodiazepine receptors. Mol Pharmacol. 1988 Dec;34(6):800–805. [PubMed] [Google Scholar]
- Wang J. K., Morgan J. I., Spector S. Benzodiazepines that bind at peripheral sites inhibit cell proliferation. Proc Natl Acad Sci U S A. 1984 Feb;81(3):753–756. doi: 10.1073/pnas.81.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Morgan J. I., Spector S. Differentiation of Friend erythroleukemia cells induced by benzodiazepines. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3770–3772. doi: 10.1073/pnas.81.12.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]


