Abstract
Protein tyrosine phosphatase δ (PTPδ) is a receptor-type PTP expressed in the specialized regions of the brain including the hippocampal CA2 and CA3, B lymphocytes and thymic medulla. To elucidate the physiological roles of PTPδ, PTPδ-deficient mice were produced by gene targeting. It was found that PTPδ-deficient mice were semi-lethal due to insufficient food intake. They also exhibited learning impairment in the Morris water maze, reinforced T-maze and radial arm maze tasks. Interestingly, although the histology of the hippocampus appeared normal, the magnitudes of long-term potentiation (LTP) induced at hippocampal CA1 and CA3 synapses were significantly enhanced in PTPδ-deficient mice, with augmented paired-pulse facilitation in the CA1 region. Thus, it was shown that PTPδ plays important roles in regulating hippocampal LTP and learning processes, and that hippocampal LTP does not necessarily positively correlate with spatial learning ability. To our knowledge, this is the first report of a specific PTP involved in the regulation of synaptic plasticity or in the processes regulating learning and memory.
Keywords: learning/long-term potentiation/semi-lethality/synaptic plasticity/tyrosine phosphatase
Introduction
Protein phosphorylation is one of the key signal transduction mechanisms that regulate cell growth, differentiation and metabolism. In particular, phosphorylation of tyrosine residues is important in signal transduction pathways downstream of various receptors including those for neurotransmitters, growth factors and cell adhesion molecules (Van Vactor, 1998). Tyrosine phosphorylation is known to be regulated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs).
We have recently cloned and characterized PTPδ, a mouse receptor-type PTP (RPTP), which is encoded by the Ptprd gene (Mizuno et al., 1993, 1994). The murine Ptprd gene is expressed in the spleen, thymus, kidney, heart and restricted regions of the brain. In situ hybridization analysis revealed that PTPδ expression in the brain is localized to the pyramidal cell layer of the hippocampal CA2–CA3 region, thalamic reticular nucleus, piriform cortex, olfactory bulb, olivary nucleus and spinal motor neurons (Mizuno et al., 1993; Sommer et al., 1997; Schaapveld et al., 1998).
In mammals, PTPδ is highly homologous to LAR (leukocyte common antigen-related protein) (Streuli et al., 1988) and PTPσ (Yan et al., 1993), forming an LAR subfamily. Highly related proteins have also been found in chicken (CRYP-α) (Stoker et al., 1995), leech (HmLAR1 and 2) (Gershon et al., 1998) and Drosophila (DLAR) (Streuli et al., 1989). All these molecules are composed of two intracellular phosphatase domains, a transmembrane domain and extracellular N-terminal immunoglobulin-like and membrane-proximal fibronectin type III domains (Brady-Kalnay and Tonks, 1995).
Essential roles of RPTP in motor axon pathfinding have been reported in Drosophila. DLAR controls the entry of specific motor axons into the correct muscle targets (Krueger et al., 1996), and DPTP69D and DPTP99A, which are also related to murine PTPδ, cooperate to allow axon defasciculation at key choice points (Desai et al., 1996). Recent gene-trap studies in mice demonstrated that LAR is involved in determining basal forebrain cholinergic neuron size and in the cholinergic innervation of the dentate gyrus (Yeo et al., 1997). PTPσ-deficient mice exhibited pituitary dysplasia and neuronal defects, suggesting that PTPσ might be involved in the development and adhesion of neuronal cells (Elchebly et al., 1999; Wallace et al., 1999). However, the role of PTPδ in murine development and physiology remains to be elucidated.
The long-term potentiation (LTP) phenomenon is a form of synaptic long-term strengthening among active neurons that represents properties initially postulated by Hebb’s theory, and it is widely believed to be a physiological basis underlying learning and memory processes (Bliss and Collingridge, 1993; Malenka, 1994). Hippocampal lesion studies and targeted gene disruption at the hippocampus suggested that the hippocampus is a critical component of the neural system that is required for the initial storage of long-term memory (Squire and Zola-Morgan, 1991; Tsien et al., 1996).
Recent studies showed that targeted gene disruption of protein kinases including CaMKII, PKCγ and Fyn causes the reduction of LTP and severe memory impairment (Grant et al., 1992; Silva et al., 1992a,b; Abeliovich et al., 1993). Involvement of PTKs in LTP induction is also shown by using specific pharmacological agents (O’Dell et al., 1991). Furthermore, tyrosine phosphorylation of N-methyl-d-aspartate (NMDA) receptor subunit 2B increases after LTP induction (Rosenblum et al., 1996), and Src family PTKs, such as Src and Fyn, activate the NMDA channel, resulting in the enhancement of LTP amplitude (Kohr and Seeburg, 1996). Moreover, Kang and Schuman (1995) suggested that BDNF and NT-3, but not NGF, are involved in LTP induction probably through modulating Trk-family receptor-type PTKs (RPTKs). These observations suggest that PTKs play an important role in regulating synaptic plasticity. Additionally, intracellular administration of a PTP inhibitor potentiates NMDA receptor-mediated currents, suggesting a role for PTPs (Wang and Salter, 1994); however, no specific PTPs have been implicated in synaptic plasticity.
To gain further insight into the physiological role of PTPs in an animal, the Ptprd gene was disrupted using gene knockout techniques. Here we show that PTPδ-deficient mice are semi-lethal and exhibit impaired spatial learning with enhanced hippocampal LTP in both the CA1 and CA3 regions. To our knowledge, this is the first report showing that a specific PTP regulates synaptic plasticity. The role of PTPδ in learning and memory as well as in regulation of LTP is discussed.
Results
Generation of Ptprd–/– mice
We have generated mice deficient in the Ptprd gene by homologous recombination. The targeting vector was constructed by replacing the signature motif of the N-terminal PTP domain with the PGKneobpA cassette (Figure 1A). After introduction of the targeting vector into embryonic stem (ES) cells, G418-resistant colonies were picked up. Three positive clones were obtained out of 1008 G418-resistant clones by screening with PCR and Southern blot hybridization. Chimeric mice were produced by the aggregation method, and germline transmission was succeeded in two independent ES cell clones (Figure 1B).
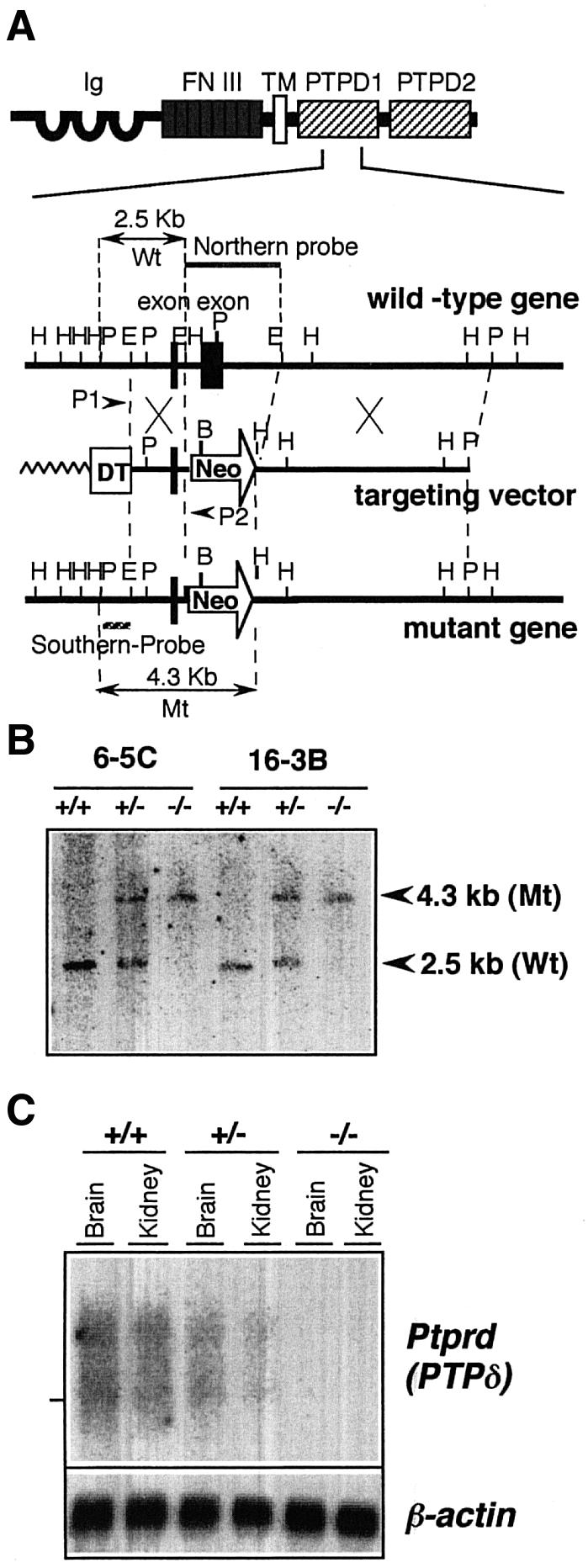
Fig. 1. Generation of PTPδ knockout mice by gene targeting. (A) The structure of PTPδ protein (upper) and the targeting strategy for the mouse genomic Ptprd locus (lower). PTPδ consists of extracellular immunoglobulin-like (Ig) and fibronectin type III-like (FN III) domains, a transmembrane domain (TM) and two PTP domains, PTPD1 and PTPD2. The exon that encodes the signature motif of PTPD1 was replaced by the neomycin resistant gene (Neo). The DT fragment was ligated at the 5′ end of the vector for negative selection. Arrowheads: PCR primers (P1 and P2). Zigzag line: plasmid DNA. Striped line: the probe for Southern blot analysis. Gray line: the probe for northern blot hybridization analysis. Black boxes: exons. Arrows: DNA fragments obtained by HindIII digestion. H: HindIII, E: EcoRI, P: PstI. (B) Southern blot hybridization analysis of the knockout mice. Genomic DNA from the liver was digested with HindIII and hybridized with the Southern probe. 6-5C and 16-3B are the targeted ES clones from which the knockout mice were derived. The expected fragment lengths for the mutant (Mt) and wild-type (Wt) alleles are indicated by arrowheads. (C) Northern blot hybridization analysis of the knockout mice. Poly(A)+ RNA was hybridized with the northern probe that detected the deleted exon. The same filter was also hybridized with a mouse β-actin probe. The position of 28S ribosomal RNA bands is indicated on the left.
Disruption of the Ptprd gene was verified by Southern blot hybridization (Figure 1B). The complete absence of the Ptprd mRNA containing the first PTP domain was confirmed by northern blot hybridization using a Ptprd-specific probe that covers the deleted sequences (Figure 1C), and by RT–PCR using a primer set that detected the deleted region (data not shown). However, we detected a shorter mRNA that corresponded to the mRNA lacking the deleted region, suggesting that a truncated mRNA lacking the phosphatase catalytic domain was expressed in Ptprd–/– mice (data not shown).
Growth retardation and semi-lethality of Ptprd–/– mice
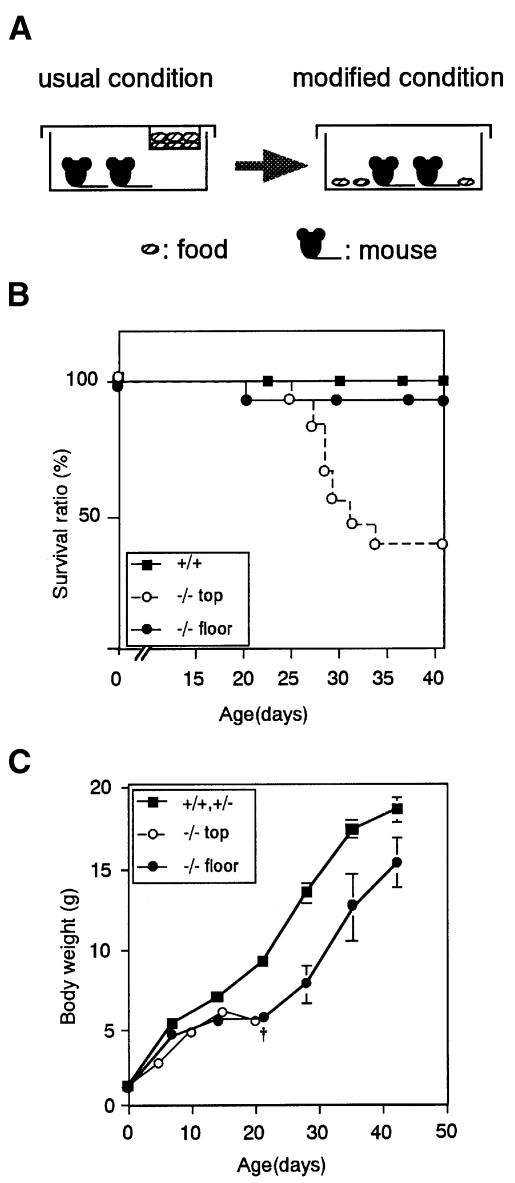
Approximately one quarter (24%) of the offspring (n = 108) from heterozygous intercrossings were found to be homozygous mutants, indicating that the homozygous mutants were viable during the embryonic stage. Homozygous mutant mice appeared normal at birth, and their body weights were not significantly different from wild-type or heterozygous mice (Figure 2C). However, under the usual feeding conditions (Figure 2A), their growth retardation was evident by day 5 and was most pronounced at 2–3 weeks of age (Figure 2C). Furthermore, 60% of Ptprd–/– mice died by day 35 (Figure 2B and C), and 40% of them survived with slightly lower body weights for at least a year. Because their loss of body weight was most notable after they were weaned, we thought that the mice might have difficulty in taking food. Indeed, once foods were placed on the floor of their cages, as shown in Figure 2A, most Ptprd–/– mice survived for a year or longer, although their body weights continued to be lower than Ptprd+/+ littermates (Figure 2B and C). These results suggest that the semi-lethality of Ptprd–/– mice is caused by starvation, probably due to some difficulties in taking food and not to an internal deficit of any kind. Both surviving male and female homozygous mutant mice were fertile.
Fig. 2. Growth retardation and semi-lethality of Ptprd–/– mice. (A) Experimental protocol. A food basket was placed on the cage cap under the usual condition (left). The food location was changed from the ceiling to the floor of the cage under the modified condition (right). (B) Semi-lethality of Ptprd–/– mice under the usual condition. The survival ratios (%) of Ptprd+/+ (n = 16) and Ptprd–/– mice (n = 11) under the usual condition, and of Ptprd–/– mice (n = 6) under the modified condition, are shown. (C) Body weights of control (+/+, +/–) (n = 10) and Ptprd–/– mice (n = 6) under the modified condition. Open circles (n = 11) show the body weight of Ptprd–/– mice under the usual condition. Under this condition, most Ptprd–/– mice died by 35 days of age as indicated by †.
Normal histology of Ptprd–/– mice
To examine possible effects of the mutation on tissue structures, we performed an extensive histological survey of the body. Although a wide variety of tissues including the heart, lung, liver, kidney, pancreas, muscle, stomach, colon, ileum, duodenum, thymus, spleen, ovary and testis from 3- and 8-week-old mice were examined with hematoxylin–eosin (HE) staining, no significant differences were detected between Ptprd+/+ and Ptprd–/– mice (data not shown). Biochemical analysis of the serum and urine also revealed no differences between the two genotypes; nor was there any abnormality in the blood cell composition of Ptprd–/– mice including their content of B220+, CD3+, CD4+ and CD8+ populations. In addition, T and B cells from the homozygous mutant mice responded equally well to mitogenic stimulation as those from Ptprd+/+ mice (data not shown).
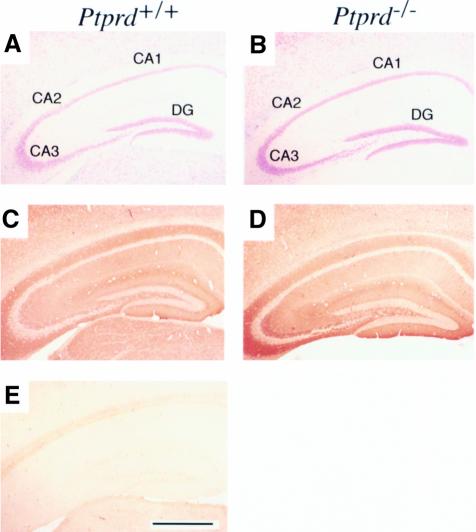
Next, we examined the histology of the brain in detail. Gross anatomy and light-microscopic observations of adult brain appeared normal in Ptprd–/– mice. When the hippocampus and other parts of the brain including the piriform cortex and thalamic reticular nucleus were examined with Klüver–Barrera (KB) stain, no abnormalities were detected (Figure 3A and B). Subsequent staining with anti-synaptophysin antibody, which reacts with a membrane component of classical, locally recycled small synaptic vesicles present in almost all synapses (Wiedenmann et al., 1986), demonstrated indistinguishable staining patterns between Ptprd+/+ and Ptprd–/– samples (Figure 3C and D). These data suggest that Ptprd gene deficiency appears to have minimal effects on the development and structure of the hippocampus, at least at a light-microscopic level.
Fig. 3. Histological analysis of Ptprd–/– mice. Hippocampus sections of 8-week-old Ptprd+/+ (A) and Ptprd–/– (B) mice were stained with the KB method. The sections were reacted with anti-synaptophysin antibody (C and D) or non-immune IgG (E). (C and E) Ptprd+/+; (D) Ptprd–/–. Hippocampal CA1, CA2, CA3 and dentate gyrus (DG) are indicated. Bar, 1 mm.
Impairment of spatial learning in Ptprd–/– mice
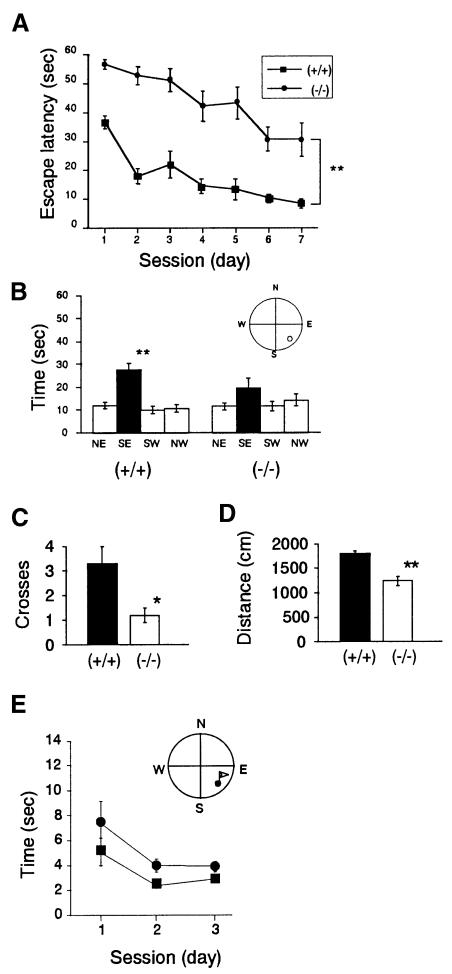
Water maze task. Since Ptprd is predominantly expressed in the hippocampus, we examined the spatial learning ability of Ptprd–/– mice using the Morris water maze task (Morris et al., 1986). First, mice were trained on a hidden platform task, in which mice searched for a submerged platform to escape from water. Ptprd–/– mice were found to take significantly longer to locate the hidden platform than Ptprd+/+ mice (Figure 4A). Nonetheless, the ability of Ptprd+/+ and Ptprd–/– mice to find the hidden platform improved over test sessions [F(6,102) = 19.821, p <0.0001], and there was no significant difference in the slopes of the learning curves [F(6,102) = 1.155, p = 0.3365]. Thus, both Ptprd+/+ and Ptprd–/– mice are able to learn the location of a hidden platform during the course of the trials, although the capacity of Ptprd–/– mice to find the platform was lower than that of Ptprd+/+ mice.
Fig. 4. Behavioral abnormalities in Ptprd–/– mice. (A) Morris water maze task. The time required to reach the hidden platform (mean ± SEM, over the seven testing days) is indicated. Ptprd+/+, n = 10; Ptprd–/–, n = 9. One-way ANOVA revealed significantly different performances between the two genotypes [F(1,17) = 27.43, **p <0.0001]. (B) The probe test after the acquisition trials of the hidden platform task. Times spent in each quadrant of the water pool are shown. SE (southeast) is the trained quadrant (closed bar). Ptprd+/+ mice spent significantly more time in the SE than in the other three quadrants (open bars) [F(3,36) = 19.10, **p <0.0001], while Ptprd–/– mice did not [F(3,32) = 2.13, p = 0.116]. (C) Number of crosses over the region where the platform was formally located (mean ± SEM) during probe test [U = 20.50, *p <0.05]. (D) Swimming distances (cm) of Ptprd+/+ (closed bar) and Ptprd–/– mice (open bar) during the probe test [t = 5.519, **p <0.0001]. (E) Escape latencies (mean ± SEM) of Ptprd+/+ (squares) and Ptprd–/– (circles) mice in the visible platform task over three testing days. One-way ANOVA revealed no significant difference between Ptprd+/+ and Ptprd–/– mice [genotype: F(1,17) = 4.19, p = 0.057].
We further performed a probe test, in which the platform was removed from the pool after completion of the hidden platform task, and the trained mice were allowed to swim freely for 60 s. Ptprd–/– mice spent time equally in all four quadrants of the pool, while Ptprd+/+ mice spent significantly longer in the target quadrant (Figure 4B). Crossings of Ptprd–/– mice over the original platform place were significantly lower than those of Ptprd+/+ mice (Figure 4C). These results indicate a spatial learning impairment in Ptprd–/– mice. The swimming distance of Ptprd–/– mice, however, was significantly shorter than that of Ptprd+/+ mice during the probe trial (Figure 4D), raising the possibility that Ptprd–/– mice may have deficits in motor function or motivation.
In the visible platform task, the performance of mice of both genotypes improved during the course of test sessions, as indicated by a progressive decrease in the latency to locate the platform (Figure 4E). The escape latency of Ptprd–/– mice tended to be longer than that of Ptprd+/+ mice, but the difference was not statistically significant. This indicates that Ptprd–/– mice could learn this visible platform task just as well as Ptprd+/+ mice.
Reinforced alternation task. To minimize the contribution made by motor ability to the behavioral evaluation of learning and memory, we carried out two additional tests: the reinforced alternation task and the radial arm maze task (Cho and Jaffard, 1995; Gerlai, 1998).
The reinforced alternation task examines whether or not mice remember the position of the reinforced arm selected in the preceding choice, irrespective of the time they required for completion (Figure 5A). Ptprd+/+ mice performed this task with 68.8% correct choices, which is well above the random chance (50%), while Ptprd–/– mice made significantly fewer correct choices (55.6%) (Figure 5C). Ptprd–/– mice tended to be hyperactive and take less time to complete one session in both sample and choice runs, but the difference was not statistically significant [F(1,17) = 3.776, p = 0.069] (Figure 5D).
Fig. 5. Defects in learning and memory in Ptprd–/– mice. (A) Diagram of the reinforced alternation task. Sliding doors (broken lines) were placed at the entrance of each arm. Each goal arm had a small well at the distal end to hold a food pellet (circles). (B) Diagram of the radial arm maze task. Food pellets were placed in a well at the tip of each maze arm (circles). (C) Reinforced alternation task. The percentage of correct arm choices is indicated. [*U(10,9) = 15.5, *p <0.05]. A broken line indicates chance level of this task. (D) Running times of sample and choice trials in reinforced alternation task. Running time per trial are represented as mean ± SEM [F(1,17) = 3.776, p = 0.069]. (E) Radial eight-arm maze task. Number of total errors made before obtaining all eight foods (mean ± SEM) is indicated [F(1,17) = 13.387, **p <0.005]. (F) Radial eight-arm maze task. Number of correct choices in the first eight trials (mean ± SEM) is shown [F(1,17) = 6.773, *p <0.05]. (G) Running time in eight-arm maze task. Running times per choice are represented as mean ± SEM [F(1,17) = 2.695, p = 0.119].
Radial arm maze task. To assess further the impairment of learning in Ptprd–/– mice, a radial arm maze was used (Figure 5B). In this task, which requires mice to remember previously visited arms in order to get rewards, error choices of Ptprd–/– mice were significantly higher than those of Ptprd+/+ mice (Figure 5E). Furthermore, when the first eight choices were compared, Ptprd–/– mice had fewer correct choices than Ptprd+/+ mice (Figure 5F). No significant differences in running time per choice were found between the two genotypes [F(1,17) = 2.695, p = 0.119] or among sessions [F(1,17) = 1.503, p = 0.173] (Figure 5G). Together with the results of the reinforced alternation task, it is reasonable to assume that Ptprd–/– mice have impaired ability in learning and memory.
Electrophysiological analysis of basal synaptic transmission and LTP
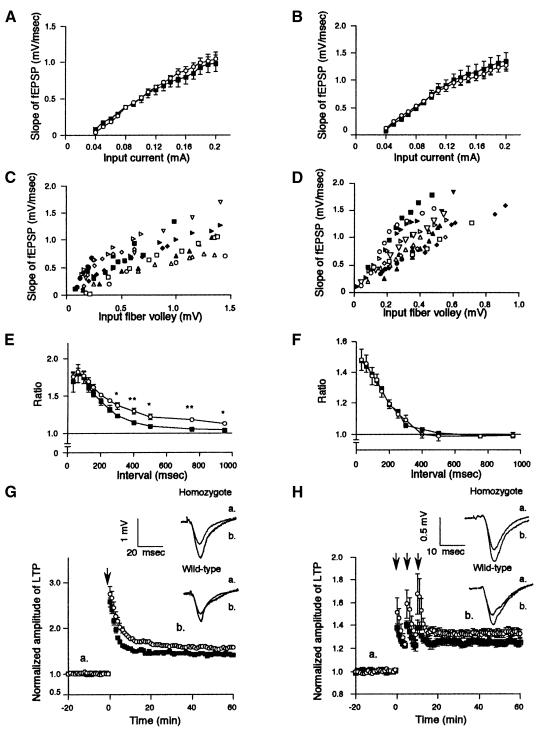
To assess the mechanism underlying these observations, we performed electrophysiological analysis of the hippocampal CA1 and CA3 regions. Excitatory synaptic responses were evoked by stimulating input fibers in the dendritic areas of CA1 and CA3 regions. The results demonstrated that in both regions, no significant alterations were observed in Ptprd–/– mice for input–output curves, the strength of stimuli (mA) versus the slope of field excitatory postsynaptic potentials (fEPSP) (mV/ms) (Figure 6A and B), or the amplitude (mV) of presynaptic fiber volley versus the slope of fEPSP (Figure 6C and D). Thus, neither the number of synapses nor basal synaptic transmission is affected in Ptprd–/– mice. Nonetheless, paired-pulse facilitation (PPF) study, in which double pulses were applied to the synaptic inputs at intervals of 30–950 ms, showed that when intervals of pulses were >300 ms, the ratio of the second responses to the first responses was significantly enhanced in the CA1 region in Ptprd–/– mice. In contrast, no significant difference was observed in the CA3 region. Thus, PTPδ seems to be involved in the repression of the synaptic transmission in the CA1 region (Figure 6E and F).
Fig. 6. Enhanced synaptic transmission in the hippocampal CA1 and CA3 regions of Ptprd–/– mice. (A and B) Input–output relationship of fEPSP slope versus stimulus intensity at the Schaffer-CA1 pyramidal cell synapses (A) and at the CA3 pyramidal cell synapses (B) in Ptprd–/– (open circles, n = 6) and Ptprd+/+ mice (closed squares, n = 6). Data are presented as mean ± SEM. (C and D) Plots of fEPSP slope versus presynaptic fiber volley amplitude at the Schaffer-CA1 pyramidal cell synapses (C) and at the CA3 pyramidal cell synapses (D) in Ptprd–/– (open symbols, n = 6) and Ptprd+/+ mice (closed symbols, n = 6). (E and F) Comparison of PPF at the Shaffer-CA1 pyramidal cell synapses (E) and at the CA3 pyramidal synapses (F) in Ptprd–/– (open circles, n = 8) and Ptprd+/+ mice (closed squares, n = 9). Data are presented as mean ± SEM (Student’s t-test, *p <0.05, **p <0.01). (G and H) Comparison of LTP induced by tetanic stimulation in Ptprd+/+ (closed squares) and Ptprd–/– mice (open circles). Tetanus was applied at the times shown by arrows. (a and b) Traces show averaged EPSP obtained at the times indicated. (G) LTP at the CA1 pyramidal cell synapses. The amplitude of LTP was calculated as the ratio of average field responses for 40 min after tetanic stimuli to the control field responses before tetanus. Data are presented as mean ± SEM. LTP induction was significantly enhanced in Ptprd–/– mice (n = 12 in Ptprd–/–, n = 10 in Ptprd+/+). (H) LTP at CA3 pyramidal cell synapses. Tetanus was applied three times as shown by arrows. In this region, the LTP was also enhanced in Ptprd–/– mice (n = 10 in both Ptprd–/– and Ptprd+/+ mice).
Finally, we examined long-term synaptic plasticity in the CA1 pyramidal neurons, which could have causal links to learning and memory. Tetanic stimulation applied to the input fibers elicited a robust increase in fEPSP followed by stable potentiation of fEPSP in Ptprd+/+ mice (+41.3 ± 4.4%). Surprisingly, the same intensity of stimulus elicited significantly enhanced LTP in Ptprd–/– mice (+56.7 ± 4.8%; p <0.01, Student’s t-test) (Figure 6G). The augmentation of LTP amplitude was similarly observed in the CA3 pyramidal neurons (Ptprd+/+, +24.4 ± 1.7%; Ptprd–/–, +32.5 ± 1.9%; p <0.05) (Figure 6H). Taken collectively, these observations suggest that PTPδ plays an important role in the modulation of long-term synaptic plasticity in the hippocampus.
Discussion
Generation of PTPδ-deficient mice
In this study, we have produced PTPδ-deficient mice by replacing the first phosphatase domain containing the catalytic center with a neomycin resistant gene. Since the invariant Cys residue within the signature motif is absolutely required for phosphatase activity (Streuli et al., 1990), these mutant mice are expected to have a negligible PTPδ phosphatase activity. Given that the extracellular and transmembrane domains were not deleted in the mutant mice, it is possible that these domains are expressed. We cannot completely exclude a possibility that this truncated gene product still plays some role in cell–cell interaction as an adhesion molecule. However, a recent report that PTPδ binds homophilically through the extracellular domain in chick (Wang and Bixby, 1999) suggests that, even if a truncated protein is expressed, this protein may not interact with other members of LAR subfamily, LAR and PTPσ. Additionally, it is unlikely that homophilic binding of PTPδ transmits signals into the cell without its PTP activity. Thus, we think that the possibility that the observed phenotypes is caused by the possible truncated gene product through interaction with other LAR subfamily members is only minimal.
Semi-lethality of Ptprd–/– mice
Homozygous PTPδ-deficient mice were born at the Mendelian ratio from crosses between heterozygous mice, suggesting that PTPδ is minimally required for survival in utero. Ptprd–/– mice, however, showed growth retardation by day 5 after birth, and under the usual feeding conditions ∼60% of them died within 6 weeks of age. The finding that Ptprd–/– mice dragged their hind legs when they walked and exhibited impaired performance in rotarod tests (T.Inoue, H.Ogura, N.Uetani, K.Kawano, M.Asano, Y.Iwakura and K.Mikoshiba, manuscript in preparation) strongly suggested that they suffered from some degree of motor dysfunction. Given that their semi-lethality was rescued by changing the position of the food, it is very likely that Ptprd–/– mice died not from internal causes but from insufficient food intake. It is of particular note that PTPσ-deficient mice also exhibited early growth retardation and neonatal mortality (Elchebly et al., 1999; Wallace et al., 1999). Minimal histological alterations may result from the complementation by the LAR-like subfamily members, PTPσ and LAR. This possibility is currently being studied.
Impaired learning and memory in Ptprd–/– mice
We found that Ptprd–/– mice have defects in learning ability, as shown in several learning tasks. Although the difference between Ptprd+/+ and Ptprd–/– mice was not clear in the Morris water maze hidden platform task, the probe test clearly suggested that spatial learning is impaired in Ptprd–/– mice. Interpretation of those data, however, is complicated by the observation that during early stages of the task, Ptprd–/– mice often rolled their body while swimming, suggesting motor dysfunction. This may explain, at least in part, why Ptprd–/– mice swam shorter distances than Ptprd+/+ mice during the probe test, and why Ptprd–/– mice tended to take longer to locate the platform in the visible platform task.
To exclude the influence of the motor dysfunction, we carried out two additional learning and memory tests: the reinforced alternative task and radial arm maze task (Cho and Jaffard, 1995; Gerlai, 1998). Since both tests assess spatial learning only by the number of correct and incorrect choices irrespective of the time required, the results should not be heavily biased by their motor ability. That the time required for a session in both tasks was even shorter, although not statistically significant, in Ptprd–/– mice suggests that motor deficit contributed minimally to the outcome of these tasks. Under these conditions, Ptprd–/– mice exhibited significantly impaired performances. Taken together, these results indicate that PTPδ is in some way involved in the regulation of spatial learning and memory.
Enhancement of hippocampal PPF in Ptprd–/– mice
It is widely accepted that hippocampus is involved in the accomplishment of water maze, reinforced alternation and radial arm maze tasks, since lesions of the hippocampal pathways lead to severe impairment of learning in these tasks (Cho and Jaffard, 1995; Logue et al., 1997; Gerlai, 1998). Our electrophysiological studies revealed no differences between Ptprd–/– and Ptprd+/+ mice in the basal transmission in either the CA1 or the CA3 region. However, we found that PPF was significantly increased in the CA1, but not the CA3 region, suggesting that PTPδ is involved in the regulation of synaptic activity in the CA1 region.
Given that PTPδ is predominantly expressed at CA2–CA3 pyramidal neurons (Mizuno et al., 1993), the enhanced PPF in the CA1 area in Ptprd–/– mice is presumably caused by a presynaptic mechanism, namely an increase in transmitter release. Moreover, it is noteworthy that the peak ratio of PPF did not change in Ptprd–/– mice (Figure 6E), but the threshold of the pulse interval causing facilitation was prolonged, indicating that while maximal enhancement of transmitter release was not changed, the decay of intracellular Ca2+ was delayed. These observations suggest that the ability to exclude intracellular Ca2+ from presynaptic sites after the first electrical stimulation is decreased in Ptprd–/– mice, causing PPF to be enhanced in the CA1 region. Consistent with this notion, Katz and Miledi (1967) suggested that enhancement of PPF is primarily caused by an increase in transmitter release evoked by the second response, which is caused by the accumulation of intracellular Ca2+ at presynaptic terminals (for a review, see Zucker, 1989).
There have been several reports suggesting that phosphorylation of tyrosine residues plays a crucial role in modulating ion channel activity. For example, Fadool and Levitan (1998) reported that K+ channel (Kv1.3) current in the olfactory bulb is suppressed by pervanadate, a PTP inhibitor, suggesting a role for PTPs in activating K+ channels. Conversely, Cataldi et al. (1996) found that PTK activation causes an increase in L-type voltage-dependent Ca2+ channel function in the pituitary GH3 cells, with PTPs exerting an inhibitory effect. The present study demonstrates for the first time that a specific PTP plays crucial roles in modulating channel activities that control intracellular Ca2+ concentration.
Augmentation of LTP in Ptprd–/– mice
The current model for the induction of LTP emphasizes the importance of activation of NMDA receptors and postsynaptic depolarization (Bliss and Collingridge, 1993). Induction of Schaffer collateral LTP at hippocampal CA1 synapses and associational–commissural fiber LTP at CA3 synapses requires activation of postsynaptic NMDA receptors. Moreover, inhibition of NMDA receptor function either by its antagonist or by disruption of NMDA receptor genes results in impairment of LTP induction as well as performance of spatial learning (Morris et al., 1986; Sakimura et al., 1995; Tsien et al., 1996). However, it should be noted that presynaptic mechanism may also be operative in the maintenance phase by increasing the transmitter release (Siegelbaum and Kandel, 1991). It has recently been reported that in addition to NMDA receptor, AMPA receptor is involved in the induction of the hippocampal LTP (Jia et al., 1996; Zamanillo et al., 1999) and that mossy fiber LTP at CA3 is independent of postsynaptic NMDA receptors and is regulated presynaptically (Zalutsky and Nicoll, 1990).
In this report, we tested LTP in the CA1 and CA3 regions of Ptprd–/– mice. The results showed that the amplitude of LTP was significantly enhanced in both regions, suggesting an inhibitory role for PTPδ in these processes. Although the precise mechanism of LTP still remains to be elucidated, it has been postulated that the induction is postsynaptic and maintenance phase is pre/postsynaptic (Siegelbaum and Kandel, 1991). Because PTPδ is expressed in the CA2–CA3 regions (Mizuno et al., 1993), augmentation of CA3 LTP in Ptprd–/– mice is consistent with a postsynaptic effect. However, the role for PTPδ in CA1 LTP could be presynaptic. PTPδ deficiency may result in the facilitation of LTP in the CA1 region due to an increase in transmitter release during tetanic stimulation, leading to a secondary increase in Ca2+ influx at postsynaptic sites through NMDA and voltage-dependent Ca2+ channels. Indeed, it has been suggested that LTP induction results in an increase in the transmitter release (Feasey et al., 1986; Malinow and Tsien, 1990). Thus, PTPδ may function in modulating LTP amplitudes both pre- and post-synaptically.
Role of protein tyrosine phosphorylation in memory formation
The data presented in this study clearly indicate that PTPδ is an important regulator of the synaptic plasticity in the hippocampus. Since the hippocampal structure of Ptprd–/– mice appeared normal at the light microscope level, it seems likely that PTPδ plays a role in regulating ion channel activity of the synapses by dephosphorylating its key substrates. Among the likely substrates are ion channels, including both pre- and post-synaptic NMDA channels, and we are now addressing this issue.
It is widely believed that LTP amplitude in the hippocampal CA1 neurons correlates with learning ability, from studies making use of both pharmacological tools and gene targeting techniques (Grant et al., 1992; Silva et al., 1992a,b; Abeliovich et al., 1993). More recently, Manabe et al. (1998) showed that in nociceptin receptor-deficient mice, enhancement of the LTP in the hippocampal CA1 neurons increases the learning ability assessed by the water maze task. In contrast to those earlier observations, we showed that LTP in the CA1 and CA3 regions is enhanced in Ptprd–/– mice, despite decreased learning ability, indicating that LTP amplitude at the hippocampus does not necessarily correlate with spatial learning ability. In this context, it is noteworthy that postsynaptic density-95-deficient mice also exhibited impaired learning in spite of enhanced CA1 LTP (Migaud et al., 1998). Those authors suggested that bi-directional synaptic plasticity is important to increase storage capacity and reduce errors for optimum memory storage. Moreover, Moser et al. (1998) found that spatial learning was impaired after saturation of LTP and suggested that synaptic plasticity is important in establishing a new memory. Other recent studies provide additional evidence dissociating LTP and learning (Gerlai, 1998; Migaud et al., 1998; Zamanillo et al., 1999).
Our observations also suggest that PTPδ deficiency-induced hyperphosphorylation of tyrosine residues of proteins in the hippocampal neurons is harmful for the acquisition of a new memory. One possible scenario is that in PTPδ-deficient mice, proteins involved in the regulation of ion channel activities of the synapses are hyperphosphorylated in all the neurons, resulting in loss of conductivity difference among synapses and setting the conductivity at elevated levels. This finally leads to a decrease in the synaptic plasticity and contributes negatively to the acquisition of a new memory. Thus, optimal synaptic potentiation needs to be tightly regulated by PTKs and PTPs, and PTPδ is one of the key enzymes in this process.
Given the extracellular structure of PTPδ, this molecule may interact with unidentified ligand(s) on a cell or extracellular matrix and this interaction may modulate the phosphatase activity. In fact, PTPβ, a member of the RPTP family, was shown to interact with such cell adhesion molecules as contactin, Tag-1 and N-CAM, which are supposed to play important roles in neuronal cell adhesion (Van Vactor, 1998). Interestingly, it was recently shown that synaptic activation induces rapid formation of input-specific dendritic spine (Engert and Bonhoeffer, 1999; Maletic-Savatic et al., 1999). Thus, it is tempting to speculate that PTPδ might be involved in cell–cell interactions or even synaptogenesis as a cell adhesion molecule, modulating the efficacy of synaptic transmission, learning and memory.
In summary, we have shown for the first time that PTPδ plays a crucial role in the CNS as a negative regulator for the neuronal activity that is important in hippocampal LTP formation. Identification of substrates and ligands of this phosphatase should provide further insight into the complex regulatory processes involved in LTP and learning.
Materials and methods
Construction of targeting vector and screening for targeted ES clones
The Ptprd gene was isolated from a 129/SvJ genomic library (Stratagene). The targeting vector was constructed by replacing the first phosphatase signature motif (2.5 kb, EcoRI–EcoRI fragment) with a PGKneobpA cassette and by ligating the diphtheria toxin A (DT) fragment DNA (Yagi et al., 1993) at the 5′ end of the vector (Figure 1A). Homologous regions at the 5′ and 3′ ends were 1.5 and 5.3 kb, respectively. The linearized targeting vector (20 µg) was electroporated (250 V, 500 µF) into 107 R1 ES cells (Nagy et al., 1993) and selected with 250 µg (active form)/ml G418 (Gibco-BRL) for 7–10 days, as previously described (Asano et al., 1997). Homologous recombinants were screened by PCR using a forward primer (P1, AATCAGAGGTGACTTCCTGTGACC) outside the targeting vector and a reverse primer (P2, GACTGCCTTGGGAAAAGCGCCTCC) in the PGKneobpA cassette. PCR was carried out for 40 cycles at 94°C for 30 s, 63°C for 60 s, 72°C for 90 s under the conditions described (Asano et al., 1997).
Generation of Ptprd–/– mice
Chimeric mice were generated using the targeted ES clones by the aggregation method (Nagy et al., 1993). Male chimeras were mated with C57BL/6J females, and homozygous mutant mice were obtained by intercrossing the heterozygous. Genotypes were determined by tail DNA dot-blot hybridization using the deleted exon DNA as a Ptprd+/+ allele-specific probe and the neo gene as a mutant allele-specific probe. Mice were kept under specific pathogen-free conditions in an environmentally controlled clean room at the Laboratory Animal Research Center, Institute of Medical Science, University of Tokyo. All experiments were conducted according to the institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments in Japan.
Southern and northern blot hybridization analysis
Genomic DNA (10 µg) prepared from the liver was digested with restriction enzymes, electrophoresed and transferred to nylon membranes (Gene Screen Plus; NEN Research Products, MA). Poly(A)+ RNA (5 µg) was prepared from the brain and kidney as previously described (Asano et al., 1997). Southern and northern hybridizations were performed according to standard methods. The 0.4 kb of PstI–EcoRI fragment and the 2.5 kb of EcoRI–EcoRI fragment were used as Southern and northern probes, respectively (Figure 1A). Mouse β-actin cDNA was used as a northern control probe.
Histological analysis
Tissues were fixed by 4% paraformaldehyde perfusion, left overnight in the same fixative, and then embedded in paraffin wax according to the standard procedure. Sections of 6 µm were made and stained with lexol fast blue and cresyl fast violet (KB stain), or with HE stain. To detect distribution of synaptophysin, the specimens were reacted with a rabbit anti-human synaptophysin antibody (DAKO, Japan) for 1 h at room temperature, followed by 10 min incubations with biotinylated anti-rabbit immunoglobulins and peroxidase-labeled streptavidin (DAKO LSAB Kit, Japan). The color reaction was developed using 3-amino-9-ethylcarbazole (AEC) as a chromogen, yielding a red-colored precipitate at the antigen site.
Learning and memory test
The behavior of male Ptprd–/– and Ptprd+/+ mice was assessed when animals reached 11 weeks of age. Experimenters were blind to the genotypes of mice tested. The mice were reused for all behavior tests in the order of the water maze, the reinforced T-maze and the radial arm maze tasks.
Water maze task
Spatial learning was assessed by three variants of the Morris water maze task (Morris et al., 1986) adapted for mice.
Hidden platform task. A circular, transparent acrylic platform (diameter 12 cm) was submerged 1 cm below the surface of the water in the center of the southeast quadrant for each trial of this task. Each mouse was allowed to swim for 60 s and the time required to reach the platform (escape latency) was recorded in each trial. In total this task consisted of four trials per day over 7 days.
Probe trial. A single probe trial was carried out after the hidden platform task had been completed. In this trial, the platform was removed and the movement of each mouse was monitored using a computer-based video tracking system (BTA-2; Muromachi Kikai Co., Ltd., Japan). Each mouse was placed into the northwest quadrant of the pool and allowed to swim for 60 s. Swim path length, the number of times crossed over the platform site and the time spent in the trained (southeast) quadrant were calculated.
Visible platform task. In this task, the circular platform was made visible by attaching a black board to it and the mouse was required to locate the visible platform. The mouse was allowed to swim for 60 s and this task consisted of four trials per day for three consecutive days.
Reinforced T-maze alternation task
The mice were housed individually with limited food, and their body weights were maintained within 80–90% of their initial values. Before testing, mice were fed with food pellets in their home cage. The tests were carried out in a T-maze constructed as described (Beracochea and Jaffard, 1995). The start box was separated from the stem by a horizontal sliding door. Sliding doors were also placed at the entrance of each arm. Each goal arm had a small well at the distal end to hold a food pellet. Following a 10 min period of exploration, mice were trained to run from the start box to one of the goal boxes to get a pellet (six trials per day for 6 days). A testing trial consisted of a sample run and a choice run. Mice were first forced to enter one of the goal arms (i.e. sample run) and immediately after consuming a pellet they were returned to the start box. The start door was opened 5 s later and mice were allowed to make a choice (i.e. choice run). During this run, the opposite arm was baited. If the animal entered the incorrect unbaited arm, it was not reinforced. Each animal received six trials per day for 8 days.
Radial arm maze task
For this task, the body weights of mice were maintained within 80–85% of their initial values. An elevated eight-arm radial maze (Hiraga and Iwasaki, 1984) adapted for a mouse was employed. Food pellets were placed in a well at the tip of each maze arm and a mouse was introduced into the central arena of the maze. The animal was left on the maze until all eight pellets were obtained or 5 min had elapsed. Each mouse received two trials per session each day for 7 days. A correct choice was recorded when the mouse entered an unvisited arm during the trial, while re-entering an arm after the food had already been obtained was counted as an error.
Electrophysiological analysis
Mice (20–28-day-old) were decapitated with a guillotine after anesthetizing with ethyl ether. Their hippocampi were rapidly dissected, cut into transverse slices (500 µm thickness) with a rotary tissue slicer at room temperature, then maintained in an incubation chamber in the presence of gassed (95% O2–5% CO2) extracellular solution containing (in mM) 124 NaCl, 3.0 KCl, 2.0 CaCl2, 2.0 MgSO4, 22.0 NaH2PO4, 1.25 NaHCO3 and 10.0 glucose, for at least 2 h at 30°C. At the time of each experiment, individual slices were transferred to a submersion recording chamber, where they were continuously perfused with extracellular solution (3 ml/min) at 30°C. Field recordings were obtained from the apical dendritic region of CA1 or CA3 using an electrode pipette (3–8 MΩ) filled with 2 M NaCl. During an experiment, the stratum radiatum was stimulated every 20 s using a concentric bipolar electrode (25 µm tips; Rhodes Medical Instruments) and 0.3 ms constant-current pulses were applied at an intensity sufficient to evoke a 50% maximal response of fEPSPs. LTP was produced by an electrical tetanic stimulation administered at 100 Hz for 1 s using the same intensity. The maximal initial negative slope of fEPSP was monitored and evaluated. Data were collected and analyzed using an IBM-based system. In all experiments, only one slice from a hemisphere was used in each individual experiment.
Statistics
The statistical significance of the data was evaluated by either two-way ANOVAs (F), one-way ANOVA (*U), Student’s t-test (t) or Mann–Whitney U-test (U). Differences were considered significant if p values were <0.05.
Acknowledgments
Acknowledgements
The authors thank Drs S.Nakamura, Y.Yoshikawa, Y.Ishihara, D.Fiorino and T.Terashima for their valuable discussions and comments. We also thank Drs A.Nagy and W.Abramow-Newerly for R1 cells, Dr T.Yagi for DT cassette, Drs K.Sudo, K.Fujimoto, A.Hoshino and S.-T.Li for their kind help in the experiments, and all the members of the laboratory for their discussions and help with animal care. This study was supported by grants from the Ministry of Education, Science, Sport and Culture of Japan, CREST, the Japan Society for the Promotion of Science, and Pioneering Research Project in Biotechnology of Japan.
References
- Abeliovich A., Paylor,R., Chen,C., Kim,J.J., Wehner,J.M. and Tonegawa,S. (1993) PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell, 75, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Asano M., Furukawa,K., Kido,M., Matsumoto,S., Umesaki,Y., Kochibe,N. and Iwakura,Y. (1997) Growth retardation and early death of β-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J., 16, 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beracochea D.J. and Jaffard,R. (1995) The effects of mammillary body lesions on delayed matching and delayed non-matching to place tasks in the mice. Behav. Brain Res., 68, 45–52. [DOI] [PubMed] [Google Scholar]
- Bliss T.V. and Collingridge,G.L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature, 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S.M. and Tonks,N.K. (1995) Protein tyrosine phosphatases as adhesion receptors. Curr. Opin. Cell Biol., 7, 650–657. [DOI] [PubMed] [Google Scholar]
- Cataldi M., Taglialatela,M., Guerriero,S., Amoroso,S., Lombardi,G., di Renzo,G. and Annunziato,L. (1996) Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. J. Biol. Chem., 271, 9441–9446. [DOI] [PubMed] [Google Scholar]
- Cho Y.H. and Jaffard,R. (1995) Spatial location learning in mice with ibotenate lesions of entorhinal cortex or subiculum. Neurobiol. Learn. Mem., 64, 285–290. [DOI] [PubMed] [Google Scholar]
- Desai C.J., Gindhart,J.G.,Jr, Goldstein,L.S. and Zinn,K. (1996) Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell, 84, 599–609. [DOI] [PubMed] [Google Scholar]
- Elchebly M., Wagner,J., Kennedy,T.E., Lanctot,C., Michaliszyn,E., Itie,A., Drouin,J. and Tremblay,M.L. (1999) Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase σ. Nature Genet., 21, 330–333. [DOI] [PubMed] [Google Scholar]
- Engert F. and Bonhoeffer,T. (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature, 399, 66–70. [DOI] [PubMed] [Google Scholar]
- Fadool D.A. and Levitan,I.B. (1998) Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J. Neurosci., 18, 6126–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasey K., Lynch,M. and Bliss,T. (1986) Long-term potentiation is associated with an increase in calcium-dependent, potassium-stimulated release of [14C]glutamate from hippocampal slices: an ex vivo study in the rat. Brain Res., 364, 39–44. [DOI] [PubMed] [Google Scholar]
- Gerlai R. (1998) A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav. Brain Res., 95, 91–101. [DOI] [PubMed] [Google Scholar]
- Gershon T.R., Baker,M.W., Nitabach,M., Wu,P. and Macagno,E.R. (1998) Two receptor tyrosine phosphatases of the LAR family are expressed in the developing leech by specific central neurons as well as select peripheral neurons, muscles and other cells. J. Neurosci., 18, 2991–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S.G., O’Dell,T.J., Karl,K.A., Stein,P.L., Soriano,P. and Kandel,E.R. (1992) Impaired long-term potentiation, spatial learning and hippocampal development in fyn mutant mice. Science, 258, 1903–1910. [DOI] [PubMed] [Google Scholar]
- Hiraga Y. and Iwasaki,T. (1984) Effects of cholinergic and monoaminergic antagonists and tranquilizers upon spatial memory in rats. Pharmacol. Biochem. Behav., 20, 205–207. [DOI] [PubMed] [Google Scholar]
- Jia Z. et al. (1996) Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron, 17, 945–956. [DOI] [PubMed] [Google Scholar]
- Kang H.J. and Schuman,E.M. (1995) Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J. Physiol. Paris, 89, 11–22. [DOI] [PubMed] [Google Scholar]
- Katz B. and Miledi,R. (1967) The timing of calcium action during neuromuscular transmission. J. Physiol. (Lond.), 189, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. and Seeburg,P.H. (1996) Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J. Physiol. (Lond.), 492, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N.X., Van Vactor,D., Wan,H.I., Gelbart,W.M., Goodman,C.S. and Saito,H. (1996) The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell, 84, 611–622. [DOI] [PubMed] [Google Scholar]
- Logue S.F., Paylor,R. and Wehner,J.M. (1997) Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci., 111, 104–113. [DOI] [PubMed] [Google Scholar]
- Malenka R.C. (1994) Synaptic plasticity in the hippocampus: LTP and LTD. Cell, 78, 535–538. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M., Malinow,R. and Svoboda,K. (1999) Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science, 283, 1923–1927. [DOI] [PubMed] [Google Scholar]
- Malinow R. and Tsien,R. (1990) Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature, 346, 177–180. [DOI] [PubMed] [Google Scholar]
- Manabe T. et al. (1998) Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature, 394, 577–581. [DOI] [PubMed] [Google Scholar]
- Migaud M. et al. (1998) Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature, 396, 433–439. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Hasegawa,K., Katagiri,T., Ogimoto,M., Ichikawa,T. and Yakura,H. (1993) MPTPδ, a putative murine homolog of HPTPδ, is expressed in specialized regions of the brain and in the B-cell lineage. Mol. Cell. Biol., 13, 5513–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Hasegawa,K., Ogimoto,M., Katagiri,T. and Yakura,H. (1994) Developmental regulation of gene expression for the MPTPδ isoforms in the central nervous system and the immune system. FEBS Lett., 355, 223–228. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Anderson,E., Lynch,G.S. and Baudry,M. (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature, 319, 774–776. [DOI] [PubMed] [Google Scholar]
- Moser E.I., Krobert,K.A., Moser,M.B. and Morris,R.G. (1998) Impaired spatial learning after saturation of long-term potentiation. Science, 281, 2038–2042. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant,J., Nagy,R., Abramow-Newerly,W. and Roder,J.C. (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl Acad. Sci. USA, 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell T.J., Kandel,E.R. and Grant,S.G. (1991) Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature, 353, 558–560. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Dudai,Y. and Richter-Levin,G. (1996) Long-term potentiation increases tyrosine phosphorylation of the N-methyl-d-aspartate receptor subunit 2B in rat dentate gyrus in vivo. Proc. Natl Acad. Sci. USA, 93, 10457–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakimura K. et al. (1995) Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε1 subunit. Nature, 373, 151–155. [DOI] [PubMed] [Google Scholar]
- Schaapveld R.Q.J., Schepens,J.T.G., Bächner,D., Attema,J., Wieringa,B., Jap,P.H.K., Hendeiksauthor,W.J.A.J. (1998) Developmental expression of the cell adhesion molecule-like protein tyrosine phosphatases LAR, RPTPδ and RPTPσ in the mouse. Mech. Dev., 77, 59–62. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S.A. and Kandel,E.R. (1991) Learning-related synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol., 1, 113–120. [DOI] [PubMed] [Google Scholar]
- Silva A.J., Stevens,C.F., Tonegawa,S. and Wang,Y. (1992a) Deficient hippocampal long-term potentiation in α-calcium-calmodulin kinase II mutant mice. Science, 257, 201–206. [DOI] [PubMed] [Google Scholar]
- Silva A.J., Paylor,R., Wehner,J.M. and Tonegawa,S. (1992b) Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science, 257, 206–211. [DOI] [PubMed] [Google Scholar]
- Sommer L., Rao,M. and Anderson,D.J. (1997) RPTPδ and the novel protein tyrosine phosphatase RPTPψ are expressed in restricted regions of the developing central nervous system. Dev. Dyn., 208, 48–61. [DOI] [PubMed] [Google Scholar]
- Squire L.R. and Zola-Morgan,S. (1991) The medial temporal lobe memory system. Science, 253, 1380–1386. [DOI] [PubMed] [Google Scholar]
- Stoker A.W., Gehrig,B., Haj,F. and Bay,B.H. (1995) Axonal localisation of the CAM-like tyrosine phosphatase CRYPα: a signalling molecule of embryonic growth cones. Development, 121, 1833–1844. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger,N.X., Hall,L.R., Schlossman,S.F. and Saito,H. (1988) A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J. Exp. Med., 168, 1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger,N.X., Tsai,A.Y. and Saito,H. (1989) A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc. Natl Acad. Sci. USA, 86, 8698–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger,N.X., Thai,T., Tang,M. and Saito,H. (1990) Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J., 9, 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J., Huerta,P. and Tonegawa,S. (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell, 87, 1327–1338. [DOI] [PubMed] [Google Scholar]
- Van Vactor D. (1998) Protein tyrosine phosphatases in the developing nervous system. Curr. Opin. Cell Biol., 10, 174–181. [DOI] [PubMed] [Google Scholar]
- Wallace M.J., Batt,J., Fladd,C.A., Henderson,J.T., Skarnes,W. and Rotin,D. (1999) Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPσ. Nature Genet., 21, 334–338. [DOI] [PubMed] [Google Scholar]
- Wang J. and Bixby,J.L. (1999) Receptor tyrosine phosphatase-δ is a homophilic, neurite-promoting cell adhesion molecule for CNS neurons. Mol. Cell. Neurosci., 14, 370–384. [DOI] [PubMed] [Google Scholar]
- Wang Y.T. and Salter,M.W. (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature, 369, 233–235. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke,W.W., Kuhn,C., Moll,R. and Gould,V.E. (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc. Natl Acad. Sci. USA, 83, 3500–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Nada,S., Watanabe,N., Tamemoto,H., Kohmura,N., Ikawa,Y. and Aizawa,S. (1993) A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal. Biochem., 214, 77–86. [DOI] [PubMed] [Google Scholar]
- Yan H., Grossman,A., Wang,H., D’Eustachio,P., Mossie,K., Musacchio,J.M., Silvennoinen,O. and Schlessinger,J. (1993) A novel receptor tyrosine phosphatase-σ that is highly expressed in the nervous system. J. Biol. Chem., 268, 24880–24886. [PubMed] [Google Scholar]
- Yeo T.T., Yang,T., Massa,S.M., Zhang,J.S., Honkaniemi,J., Butcher,L.L. and Longo,F.M. (1997) Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J. Neurosci. Res., 47, 348–360. [DOI] [PubMed] [Google Scholar]
- Zalutsky R.A. and Nicoll,R.A. (1990) Comparison of two forms of long-term potentiation in single hippocampal neurons. Science, 248, 1619–1624. [DOI] [PubMed] [Google Scholar]
- Zamanillo D. et al. (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science, 284, 1805–1811. [DOI] [PubMed] [Google Scholar]
- Zucker R.S. (1989) Short-term synaptic plasticity. Annu. Rev. Neurosci., 12, 13–31. [DOI] [PubMed] [Google Scholar]