Abstract
Tumours with necrotic regions have an inadequate blood supply and are expected to differ from well-vascularised tumours in response to treatment. The purpose of the present work was to investigate whether proton magnetic resonance imaging (MRI) might be used to detect necrotic regions in tumours. MR images and histological sections from individual tumours of three different amelanotic human melanoma xenograft lines (BEX-t, HUX-t, SAX-t) were analysed in pairs. MRI was performed at 1.5 T using two spin-echo pulse sequences, one with a repetition time (TR) of 600 ms and echo times (TEs) of 20, 40, 60 and 80 ms and the other with a TR of 2000 ms and TEs of 20, 40, 60 and 80 ms. Spin-lattice relaxation time (T1), spin-spin relaxation time (T2) and proton density (N0) were calculated for each volume element corresponding to a pixel. Synthetic MR images, pure T1, T2 and N0 images and spin-echo images with chosen values for TR and TE were generated from these data. T1, T2 and N0 distributions of tumour subregions, corresponding to necrotic regions and regions of viable tissue as defined by histological criteria, were also generated. T1 and T2 were significantly shorter in the necrotic regions than in the regions of viable tissue in all tumours. These differences were sufficiently large to allow the generation of synthetic spin-echo images showing clear contrast between necrosis and viable tissue. Maximum contrast was achieved with TRs within the range 2800-4000 ms and TEs within the range 160-200 ms. Necrotic tissue could also be distinguished from viable tissue in pure T1 and T2 images. Consequently, the possibility exists that MRI might be used for detection of necrotic regions in tumours and hence for prediction of tumour treatment response.
Full text
PDF
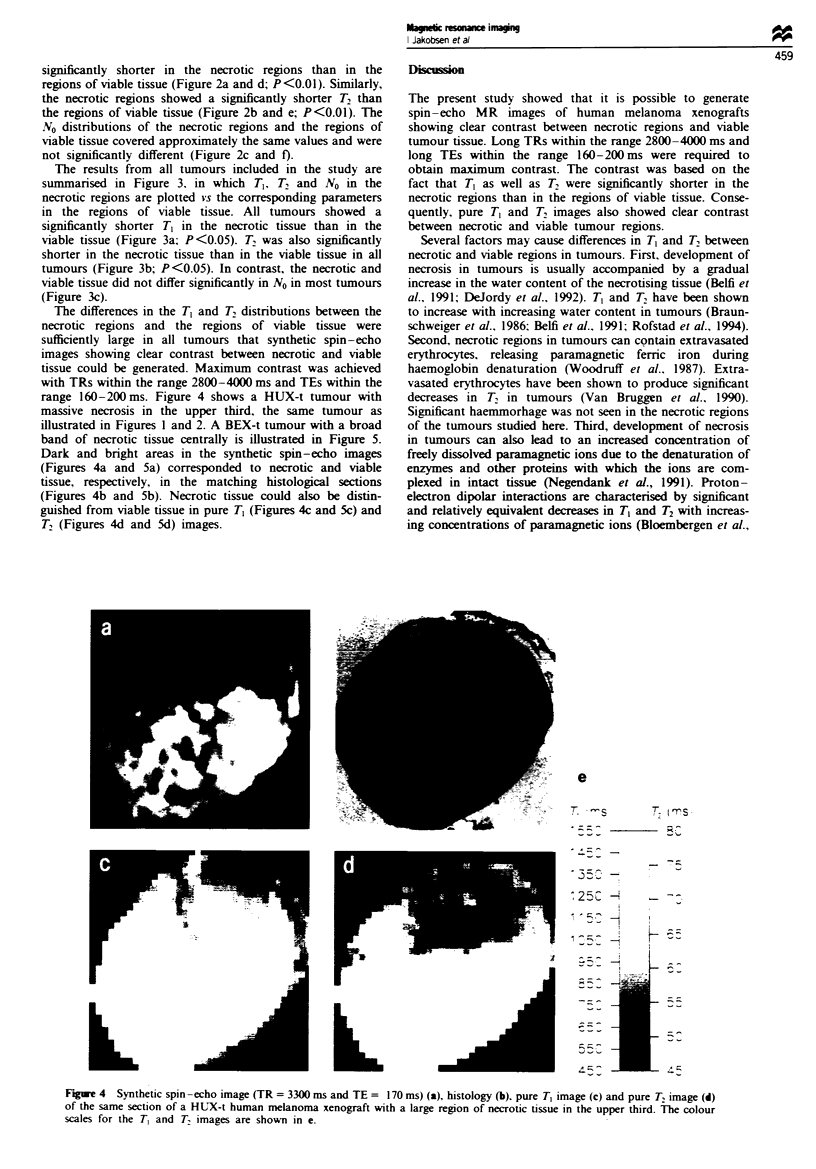
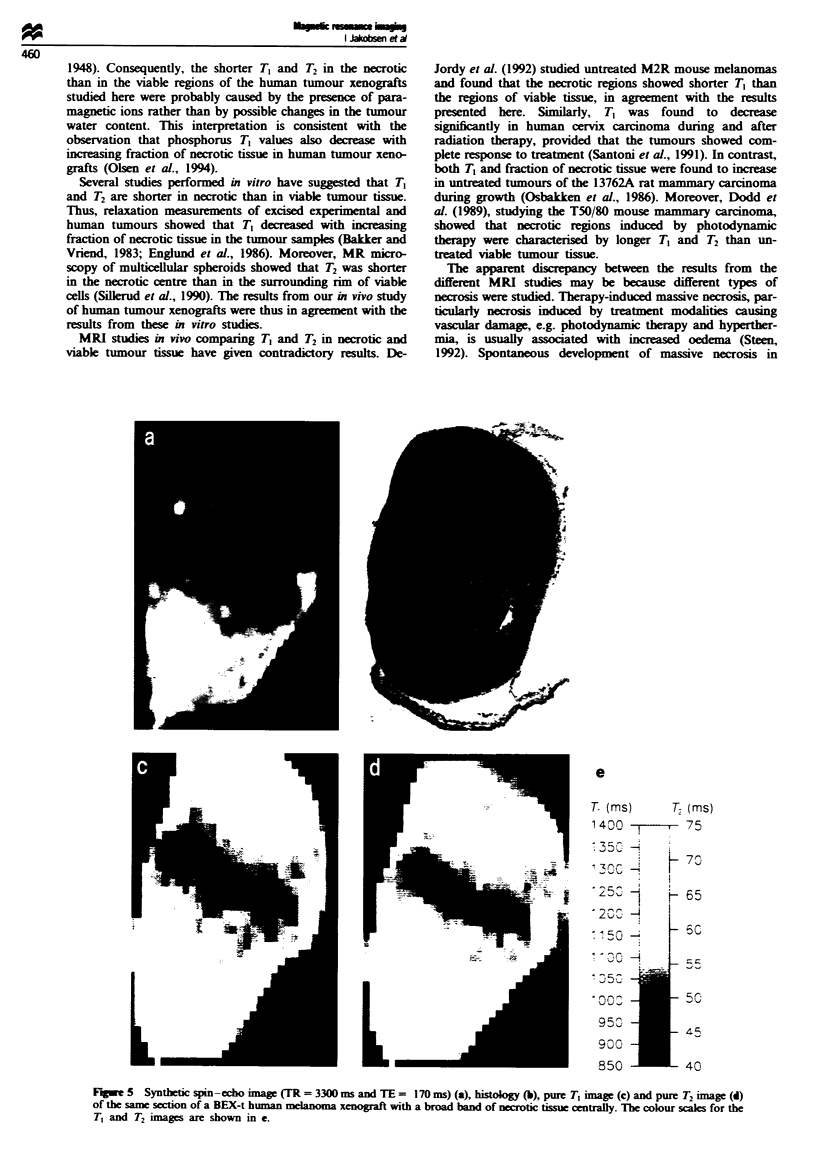

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker C. J., Vriend J. Proton spin-lattice relaxation studies of tissue response to radiotherapy in mice. Phys Med Biol. 1983 Apr;28(4):331–340. doi: 10.1088/0031-9155/28/4/001. [DOI] [PubMed] [Google Scholar]
- Belfi C. A., Medendorp S. V., Ngo F. Q. The response of the KHT sarcoma to radiotherapy as measured by water proton NMR relaxation times: relationships with tumor volume and water content. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):497–507. doi: 10.1016/0360-3016(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Braunschweiger P. G., Schiffer L. M., Furmanski P. 1H-NMR relaxation times and water compartmentalization in experimental tumor models. Magn Reson Imaging. 1986;4(4):335–342. doi: 10.1016/0730-725x(86)91043-x. [DOI] [PubMed] [Google Scholar]
- Chapman J. D. Measurement of tumor hypoxia by invasive and non-invasive procedures: a review of recent clinical studies. Radiother Oncol. 1991;20 (Suppl 1):13–19. doi: 10.1016/0167-8140(91)90181-f. [DOI] [PubMed] [Google Scholar]
- DeJordy J. O., Bendel P., Horowitz A., Salomon Y., Degani H. Correlation of MR imaging and histologic findings in mouse melanoma. J Magn Reson Imaging. 1992 Nov-Dec;2(6):695–700. doi: 10.1002/jmri.1880020614. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Review article: angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993 Mar;66(783):181–196. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- Dodd N. J., Moore J. V., Poppitt D. G., Wood B. In vivo magnetic resonance imaging of the effects of photodynamic therapy. Br J Cancer. 1989 Aug;60(2):164–167. doi: 10.1038/bjc.1989.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Phelps M. E. PET in clinical oncology. Cancer Metastasis Rev. 1988 Jun;7(2):119–142. doi: 10.1007/BF00046482. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Negendank W., Corbett T., Crowley M., Kellogg C. Evidence for a contribution of paramagnetic ions to water proton spin-lattice relaxation in normal and malignant mouse tissues. Magn Reson Med. 1991 Apr;18(2):280–293. doi: 10.1002/mrm.1910180204. [DOI] [PubMed] [Google Scholar]
- Olsen D. R., Lyng H., Southon T. E., Rofstad E. K. 31P-nuclear magnetic resonance spectroscopy in vivo of four human melanoma xenograft lines: spin-lattice relaxation times. Radiother Oncol. 1994 Jul;32(1):54–62. doi: 10.1016/0167-8140(94)90449-9. [DOI] [PubMed] [Google Scholar]
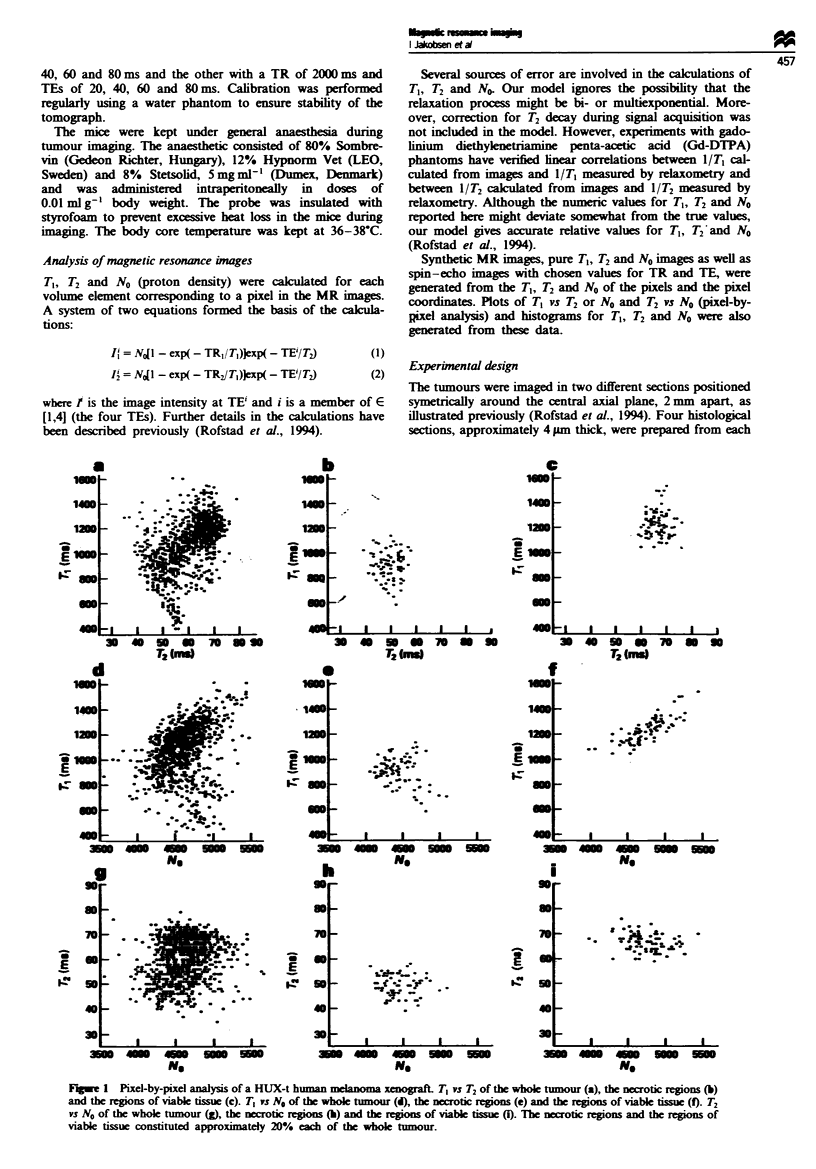
- Rofstad E. K., Steinsland E., Kaalhus O., Chang Y. B., Høvik B., Lyng H. Magnetic resonance imaging of human melanoma xenografts in vivo: proton spin-lattice and spin-spin relaxation times versus fractional tumour water content and fraction of necrotic tumour tissue. Int J Radiat Biol. 1994 Mar;65(3):387–401. doi: 10.1080/09553009414550451. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Wahl A., Stokke T., Nesland J. M. Establishment and characterization of six human melanoma xenograft lines. APMIS. 1990 Oct;98(10):945–953. doi: 10.1111/j.1699-0463.1990.tb05019.x. [DOI] [PubMed] [Google Scholar]
- Santoni R., Bucciolini M., Chiostrini C., Cionini L., Renzi R. Quantitative magnetic resonance imaging in cervical carcinoma: a report on 30 cases. Br J Radiol. 1991 Jun;64(762):498–504. doi: 10.1259/0007-1285-64-762-498. [DOI] [PubMed] [Google Scholar]
- Sillerud L. O., Freyer J. P., Neeman M., Mattingly M. A. Proton NMR microscopy of multicellular tumor spheroid morphology. Magn Reson Med. 1990 Dec;16(3):380–389. doi: 10.1002/mrm.1910160304. [DOI] [PubMed] [Google Scholar]
- Steen R. G. Edema and tumor perfusion: characterization by quantitative 1H MR imaging. AJR Am J Roentgenol. 1992 Feb;158(2):259–264. doi: 10.2214/ajr.158.2.1729777. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Rasey J. S., Hill R. P. Tumor biology. Am J Clin Oncol. 1988 Jun;11(3):253–274. doi: 10.1097/00000421-198806000-00004. [DOI] [PubMed] [Google Scholar]
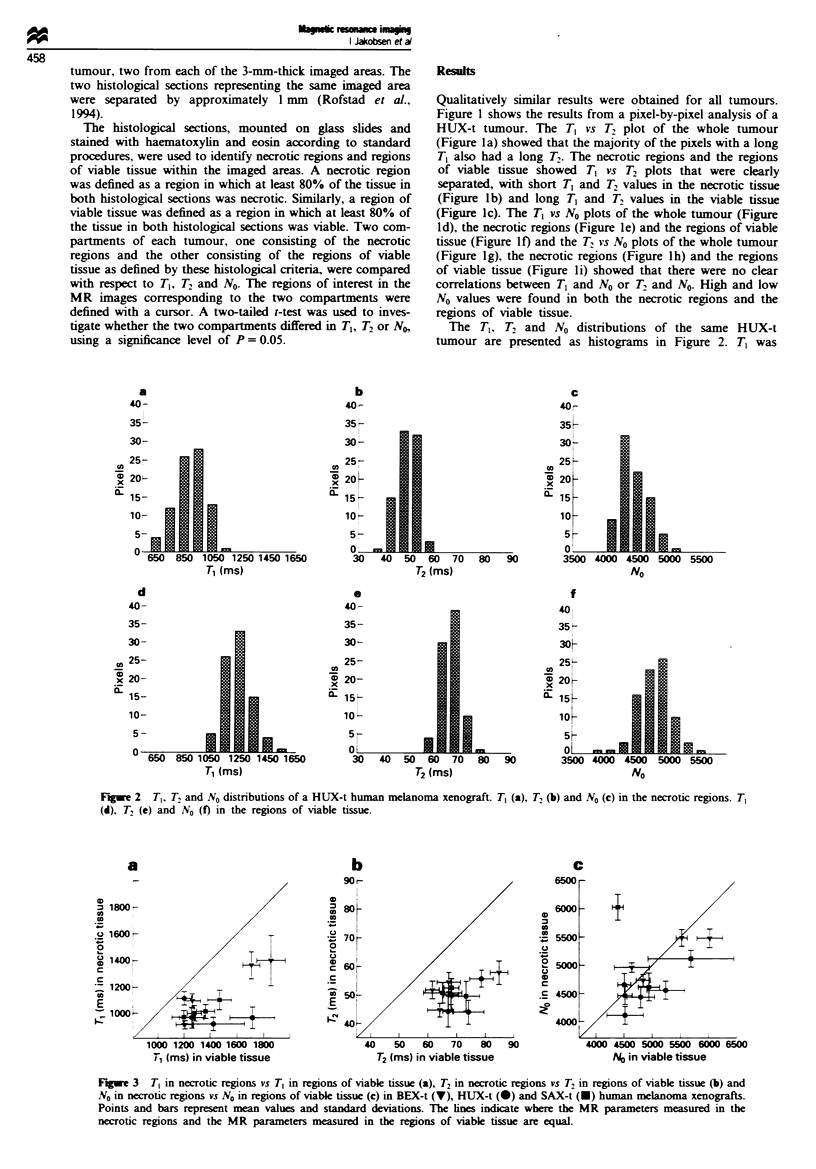
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bruggen N., Syha J., Busza A. L., King M. D., Stamp G. W., Williams S. R., Gadian D. G. Identification of tumor hemorrhage in an animal model using spin echoes and gradient echoes. Magn Reson Med. 1990 Jul;15(1):121–127. doi: 10.1002/mrm.1910150113. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Woodruff W. W., Jr, Djang W. T., McLendon R. E., Heinz E. R., Voorhees D. R. Intracerebral malignant melanoma: high-field-strength MR imaging. Radiology. 1987 Oct;165(1):209–213. doi: 10.1148/radiology.165.1.3628773. [DOI] [PubMed] [Google Scholar]