Abstract
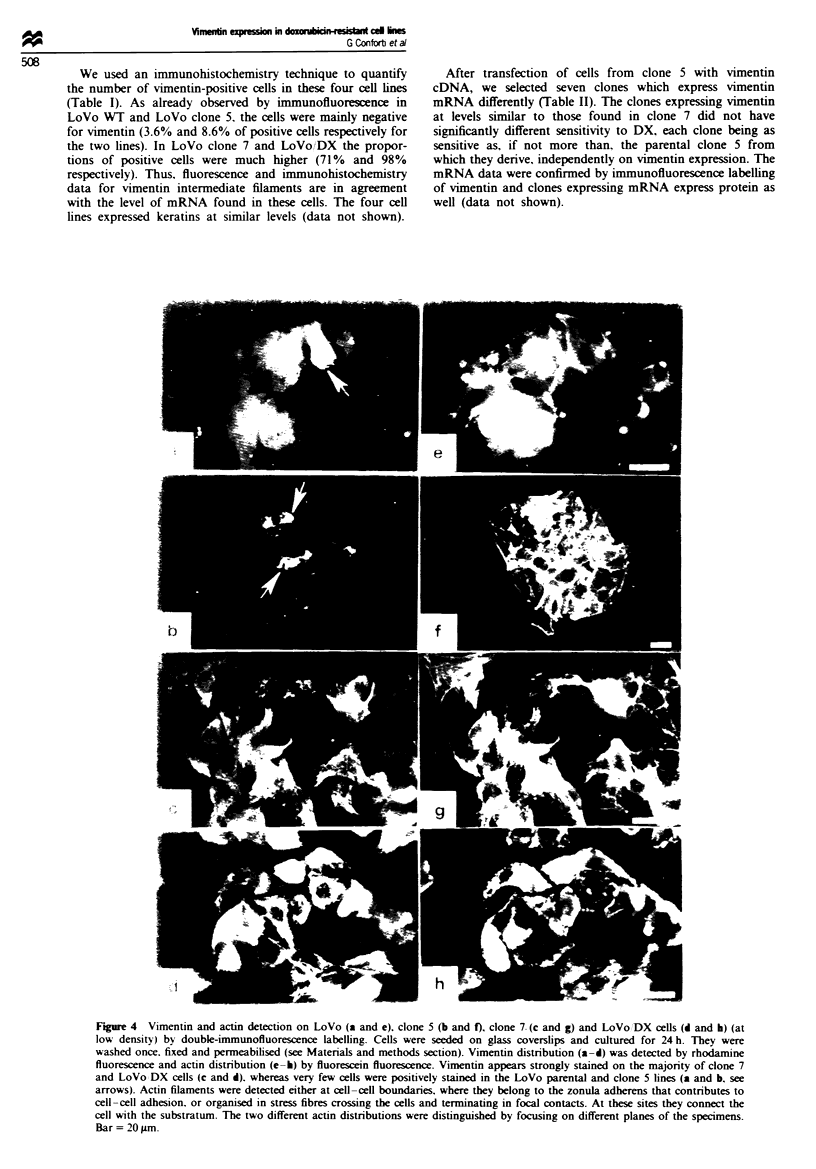
We selected two clones, isolated from the human colocarcinoma cell line LoVo, showing a sensitivity to doxorubicin similar to (LoVo clone 5) or three times lower than (LoVo clone 7) the parental cell line. Since vimentin was atypically expressed in a human breast carcinoma cell line made resistant to doxorubicin, we looked at vimentin expression in these two clones with spontaneously different sensitivity to the drug. For comparison we used the parental cell line LoVo WT and LoVo/DX made resistant pharmacologically. mRNA for vimentin was undetectable by Northern blot analysis in LoVo WT and in LoVo clone 5, while expression of this gene was high in LoVo clone 7 and in LoVo/DX. This increase in mRNA levels was not related to an amplification of DNA, as suggested by Southern blot analysis. Immunofluorescence and immunocytochemistry findings confirmed, at protein level, the mRNA data. In LoVo clones 5 and 7, there were respectively 8.6% and 71% vimentin-positive cells, although the two clones showed similar expression of multidrug resistance gene 1 (mdr-1) and accumulated intracellular doxorubicin at similar levels. Similarly, drug efflux was the same for both clones. Our results show for the first time that cells resistant to doxorubicin express vimentin independently of the mdr glycoprotein. However when cells from clone 5 were transfected with human vimentin cDNA, they did not become resistant, indicating that vimentin can be considered as a marker of resistance in these cells but does not give rise to a resistant phenotype by itself.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aller P., Rius C., Mata F., Zorrilla A., Cabañas C., Bellón T., Bernabeu C. Camptothecin induces differentiation and stimulates the expression of differentiation-related genes in U-937 human promonocytic leukemia cells. Cancer Res. 1992 Mar 1;52(5):1245–1251. [PubMed] [Google Scholar]
- Aubin J. E., Osborn M., Franke W. W., Weber K. Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp Cell Res. 1980 Sep;129(1):149–165. doi: 10.1016/0014-4827(80)90340-7. [DOI] [PubMed] [Google Scholar]
- Broggini M., Grandi M., Ubezio P., Geroni C., Giuliani F. C., D'Incalci M. Intracellular doxorubicin concentrations and drug-induced DNA damage in a human colon adenocarcinoma cell line and in a drug-resistant subline. Biochem Pharmacol. 1988 Dec 1;37(23):4423–4431. doi: 10.1016/0006-2952(88)90656-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. P., Chanda E. R., Dicke F. P., Gerlach J. H., Mirski S. E. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991 Jul 1;51(13):3345–3352. [PubMed] [Google Scholar]
- Dolfini E., Dasdia T., Perletti G., Romagnoni M., Piccinini F. Analysis of calcium-dependent protein kinase-C isoenzymes in intrinsically resistant cloned lines of LoVo cells: reversal of resistance by kinase inhibitor 1-(5-isoquinolinylsulfonyl) 2-methylpiperazine. Anticancer Res. 1993 Jul-Aug;13(4):1123–1127. [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982 May;29(1):43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Farrell F. X., Sax C. M., Zehner Z. E. A negative element involved in vimentin gene expression. Mol Cell Biol. 1990 May;10(5):2349–2358. doi: 10.1128/mcb.10.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Narni F., Mars W., Kaczmarek L., Venturelli D., Anderson B., Calabretta B. Expression of growth-regulated genes in human acute leukemias. Cancer Res. 1986 Oct;46(10):5162–5166. [PubMed] [Google Scholar]
- Ford J. M., Hait W. N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990 Sep;42(3):155–199. [PubMed] [Google Scholar]
- Franke W. W., Grund C., Kuhn C., Lehto V. P., Virtanen I. Transient change of organization of vimentin filaments during mitosis as demonstrated by a monoclonal antibody. Exp Cell Res. 1984 Oct;154(2):567–580. doi: 10.1016/0014-4827(84)90181-2. [DOI] [PubMed] [Google Scholar]
- Grandi M., Geroni C., Giuliani F. C. Isolation and characterization of a human colon adenocarcinoma cell line resistant to doxorubicin. Br J Cancer. 1986 Sep;54(3):515–518. doi: 10.1038/bjc.1986.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Chu Y. W., Seftor R. E., Nagle R. B., McDaniel K. M., Leong S. P., Yohem K. H., Leibovitz A. M., Meyskens F. L., Jr Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992 Feb 5;84(3):165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- Hennekes H., Kühn S., Traub P. Coding sequence and flanking regions of the mouse vimentin gene. Mol Gen Genet. 1990 Mar;221(1):33–36. doi: 10.1007/BF00280364. [DOI] [PubMed] [Google Scholar]
- Järvinen M. Vimentin in human erythroleukemia (HEL) cells is modulated with differentiation inducers. Cell Biol Int Rep. 1990 Mar;14(3):199–209. doi: 10.1016/s0309-1651(05)80002-5. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A., Paulin D. Activation of the human vimentin gene by the Tax human T-cell leukemia virus. I. Mechanisms of regulation by the NF-kappa B transcription factor. J Biol Chem. 1993 Jan 25;268(3):2180–2188. [PubMed] [Google Scholar]
- Paine M. L., Gibbins J. R., Chew K. E., Demetriou A., Kefford R. F. Loss of keratin expression in anaplastic carcinoma cells due to posttranscriptional down-regulation acting in trans. Cancer Res. 1992 Dec 1;52(23):6603–6611. [PubMed] [Google Scholar]
- Paulin-Levasseur M., Giese G., Scherbarth A., Traub P. Expression of vimentin and nuclear lamins during the in vitro differentiation of human promyelocytic leukemia cells HL-60. Eur J Cell Biol. 1989 Dec;50(2):453–461. [PubMed] [Google Scholar]
- Salvetti A., Lilienbaum A., Li Z., Paulin D., Gazzolo L. Identification of a negative element in the human vimentin promoter: modulation by the human T-cell leukemia virus type I Tax protein. Mol Cell Biol. 1993 Jan;13(1):89–97. doi: 10.1128/mcb.13.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Heckford S. E., Skerker J. M., Worland P., Torri J. A., Thompson E. W., Byers S. W., Gelmann E. P. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992 Oct 1;52(19):5190–5197. [PubMed] [Google Scholar]
- Sommers C. L., Walker-Jones D., Heckford S. E., Worland P., Valverius E., Clark R., McCormick F., Stampfer M., Abularach S., Gelmann E. P. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989 Aug 1;49(15):4258–4263. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stover D. M., Zehner Z. E. Identification of a cis-acting DNA antisilencer element which modulates vimentin gene expression. Mol Cell Biol. 1992 May;12(5):2230–2240. doi: 10.1128/mcb.12.5.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimi M., Château M. T., Marti J., Pacaud M. Induction of differentiation of the human histiocytic lymphoma cell line U937 in the absence of vimentin expression. Differentiation. 1990 Oct;45(1):55–60. doi: 10.1111/j.1432-0436.1990.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Tsuru A., Nakamura N., Takayama E., Suzuki Y., Hirayoshi K., Nagata K. Regulation of the expression of vimentin gene during the differentiation of mouse myeloid leukemia cells. J Cell Biol. 1990 May;110(5):1655–1664. doi: 10.1083/jcb.110.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino F., Capranico G. DNA topoisomerase II as the primary target of anti-tumor anthracyclines. Anticancer Drug Des. 1990 Nov;5(4):307–317. [PubMed] [Google Scholar]
- van de Klundert F. A., van Eldik G. J., Pieper F. R., Jansen H. J., Bloemendal H. Identification of two silencers flanking an AP-1 enhancer in the vimentin promoter. Gene. 1992 Dec 15;122(2):337–343. doi: 10.1016/0378-1119(92)90223-c. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]








