Abstract
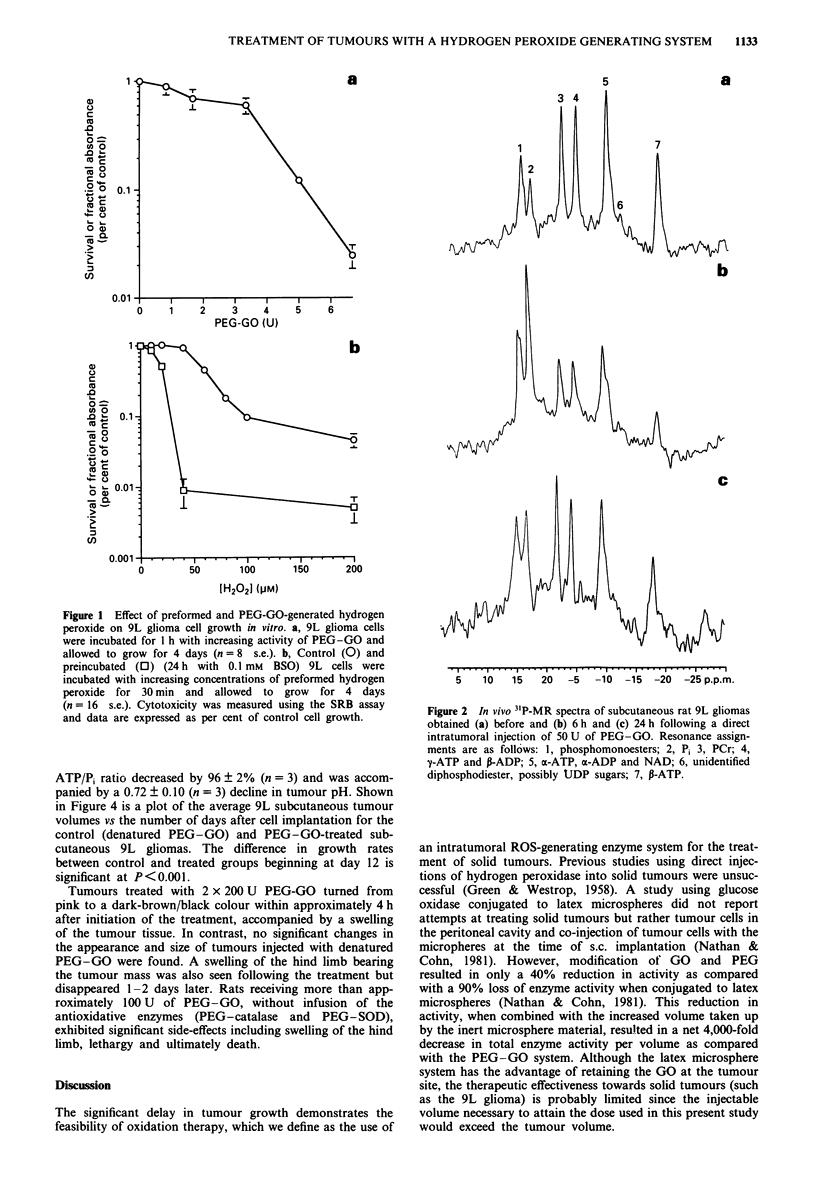
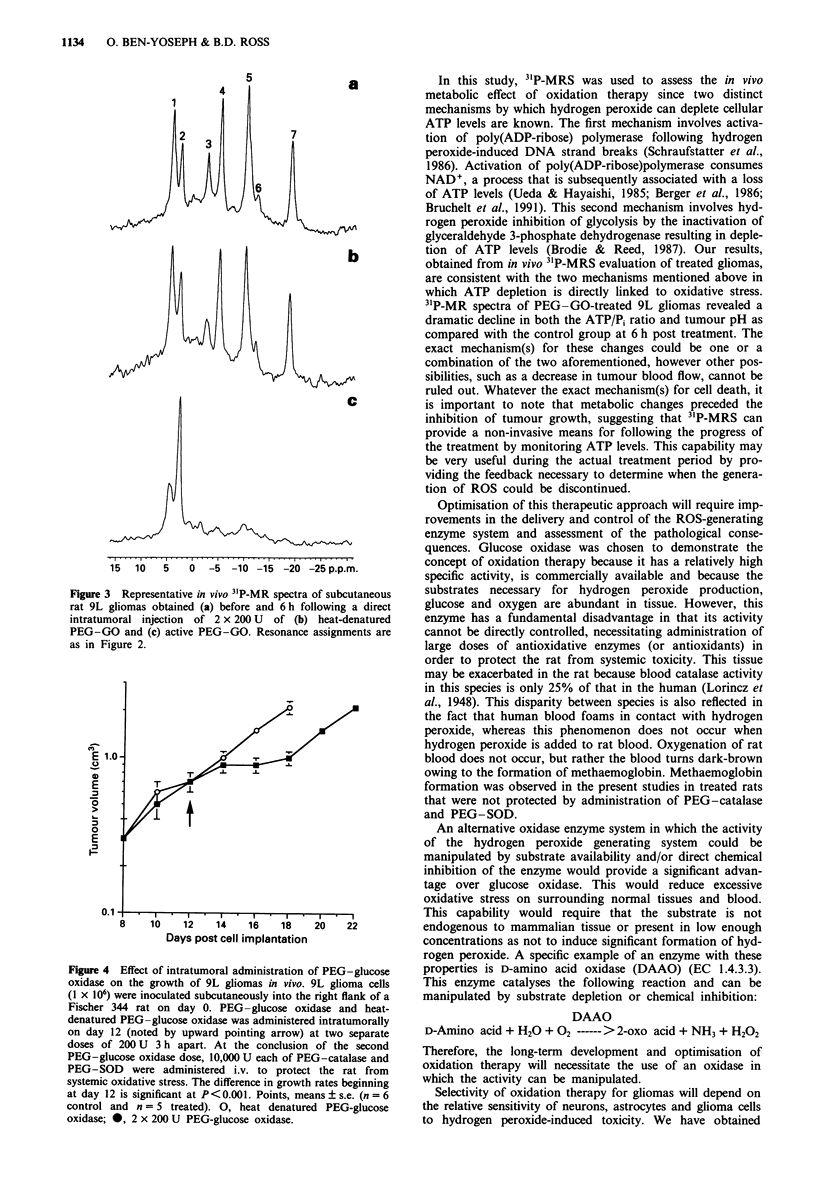
Oxygen radicals induce cytotoxicity via a variety of mechanisms, including DNA damage, lipid peroxidation and protein oxidation. Here, we explore the use of a polyethylene glycol (PEG)-stabilised enzyme capable of producing reactive oxygen species (ROS), glucose oxidase (GO), for the purpose of harnessing the cytotoxic potential of ROS for treating solid tumours. PEG-GO (200 U), administered by two intratumoral injections 3 h apart, produced a significant growth delay in subcutaneous rat 9L gliomas as compared with control animals receiving heat-denatured PEG-GO. Rats were protected from systemic toxicity by subsequent i.v. administration of PEG-superoxide dismutase (PEG-SOD) and PEG-catalase. In vivo tumour metabolic changes, monitored using 31P magnetic resonance spectroscopy (31P-MRS) 6 h following initial administration of PEG-GO, revealed a 96 +/- 2% reduction in the ATP/Pi ratio and a 0.72 +/- 0.10 unit decline in intracellular pH. A 3-fold sensitisation of 9L glioma cells in vitro to hydrogen peroxide could be achieved by a 24 h preincubation with buthionine sulphoximine (BSO). This study suggests that oxidation therapy, the use of an intratumoral ROS-generating enzyme system for the treatment of solid tumours, is a promising area which warrants further exploration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger N. A. Cancer chemotherapy: new strategies for success. J Clin Invest. 1986 Nov;78(5):1131–1135. doi: 10.1172/JCI112692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O., Erickson L. C. Comparison of the effects of hydrogen peroxide and x-ray irradiation on toxicity, mutation, and DNA damage/repair in mammalian cells (V-79). Biochim Biophys Acta. 1981 Jun 26;654(1):135–141. doi: 10.1016/0005-2787(81)90146-5. [DOI] [PubMed] [Google Scholar]
- Brodie A. E., Reed D. J. Reversible oxidation of glyceraldehyde 3-phosphate dehydrogenase thiols in human lung carcinoma cells by hydrogen peroxide. Biochem Biophys Res Commun. 1987 Oct 14;148(1):120–125. doi: 10.1016/0006-291x(87)91084-9. [DOI] [PubMed] [Google Scholar]
- Bruchelt G., Schraufstätter I. U., Niethammer D., Cochrane C. G. Ascorbic acid enhances the effects of 6-hydroxydopamine and H2O2 on iron-dependent DNA strand breaks and related processes in the neuroblastoma cell line SK-N-SH. Cancer Res. 1991 Nov 15;51(22):6066–6072. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- HOLLCROFT J. W., LORENZ E., MATTHEWS M. Factors modifying the effect of x-irradiation on regression of a transplanted lymphosarcoma. J Natl Cancer Inst. 1952 Feb;12(4):751–763. [PubMed] [Google Scholar]
- Hoffmann M. E., Meneghini R. Action of hydrogen peroxide on human fibroblast in culture. Photochem Photobiol. 1979 Jul;30(1):151–155. doi: 10.1111/j.1751-1097.1979.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Kaibara N., Ikeda T., Hattori T., Inokuchi K. Experimental studies on enhancing the therapeutic effect of mitomycin-C with hydrogen peroxide. Jpn J Exp Med. 1971 Aug;41(4):323–329. [PubMed] [Google Scholar]
- Lippitz B. E., Halperin E. C., Griffith O. W., Colvin O. M., Honore G., Ostertag C. B., Bigner D. D., Friedman H. S. L-buthionine-sulfoximine-mediated radiosensitization in experimental interstitial radiotherapy of intracerebral D-54 MG glioma xenografts in athymic mice. Neurosurgery. 1990 Feb;26(2):255–260. doi: 10.1097/00006123-199002000-00012. [DOI] [PubMed] [Google Scholar]
- MAKINO S., TANAKA T. The cytological effects of chemicals on ascites sarcomas. II. Selective damage to tumor cells by CaCl2, AlCl3 and H2O21. Gan. 1953 Mar;44(1):39–46. [PubMed] [Google Scholar]
- Madden A., Leach M. O., Collins D. J., Payne G. S. The water resonance as an alternative pH reference: relevance to in vivo 31P NMR localized spectroscopy studies. Magn Reson Med. 1991 Jun;19(2):416–421. doi: 10.1002/mrm.1910190232. [DOI] [PubMed] [Google Scholar]
- Mealey J., Jr Regional infusion of vinblastine and hydrogen peroxide in tumor-bearing rats. Cancer Res. 1965 Dec;25(11):1839–1843. [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Michiels C., Remacle J. Use of the inhibition of enzymatic antioxidant systems in order to evaluate their physiological importance. Eur J Biochem. 1988 Nov 1;177(2):435–441. doi: 10.1111/j.1432-1033.1988.tb14393.x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Cohn Z. A. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981 Nov 1;154(5):1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott G. W., Kulczycki A., Jr, Grass E. H., Parker C. W. Selective binding and cytotoxicity of rat basophilic leukemia cells (RBL-1) with immunoglobulin E-biotin and avidin-glucose oxidase conjugates. J Immunol. 1980 Sep;125(3):1201–1209. [PubMed] [Google Scholar]
- Pyatak P. S., Abuchowski A., Davis F. F. Preparation of a polyethylene glycol: superoxide dismutase adduct, and an examination of its blood circulation life and anti-inflammatory activity. Res Commun Chem Pathol Pharmacol. 1980 Jul;29(1):113–127. [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. V., Shoemaker R. H., Paull K. D., Simon R. M., Tosini S., Skehan P., Scudiero D. A., Monks A., Boyd M. R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990 Jul 4;82(13):1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- SUGIURA K. Effect of hydrogen peroxide on transplanted rat and mouse tumours. Nature. 1958 Nov 8;182(4645):1310–1311. doi: 10.1038/1821310a0. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER F. C. Studies with various experimental tumors. Cancer Res. 1953;13(Suppl 1):81–89. [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]



