Abstract
Cdc7p is a protein kinase that is required for G1/S transition and initiation of DNA replication in Saccharomyces cerevisiae. The mechanisms whereby Cdc7p and its substrates exerts their effects are unknown. We report here the characterization in S. cerevisiae of a recessive mutation in a member of the MCM family, MCM5/CDC46, which bypasses the requirement for Cdc7p and its interacting factor Dbf4p. Because the MCM family of evolutionarily conserved proteins have been implicated in restricting DNA replication to once per cell cycle, our studies suggest that Cdc7p is required late in G1 because in its absence the Mcm5p/Cdc46p blocks the initiation of DNA replication. Moreover, Mcm5p/Cdc46p may have both positive and negative effects on the ability of cell to initiate replication.
Eukaryotic cells strictly limit the duplication of their genome. DNA replication initiates from sequences called origins no more than once per cell cycle. This regulation of origin usage results in the precise duplication of each of the cell’s chromosomes and a block to rereplication until the cell segregates its chromosomes during mitosis.
An evolutionarily conserved family of genes, the MCM family are required for DNA replication (1–3). In Saccharomyces cerevisiae, the family of MCM proteins is comprised of six members including the Mcm2p (4, 5), Mcm3p (5), Mcm4p/Cdc54p (6), Mcm5p/Cdc46p (7, 8), Mcm6p (identified by the yeast genome project), and Mcm7p/Cdc47p (9, 10) all of which share a highly conserved 200-amino acid region (1, 2). Using Xenopus egg extracts, the MCM proteins have been linked biochemically to an activity which is able to modify or license replication–incompetent chromatin to a replication–competent state (11). Localization of the MCM proteins in yeast are cell cycle-regulated and are detected in the nucleus only from late in mitosis to early in S phase (5, 10, 12). While in the nucleus during G1 the MCM proteins appear to be bound to chromatin (5). In mammalian and Xenopus cells, MCM proteins are present in the nucleus constitutively and the binding of the MCM proteins to chromatin is restricted to late in mitosis to early in S phase as DNA replication progresses (13–16).
Two evolutionarily conserved kinases, the cyclin B(Clb)/cyclin-dependent kinase (Cdk) and Cdc7p, are required late in G1 to initiate DNA replication (3). The phosphorylation of Mcm4p by Clb/Cdk appears to play a role in triggering the removal of the MCM proteins from chromatin (17). However the substrates and the mechanisms for the essential functions of Cdc7p are unknown. CDC7 is not required for premeiotic DNA replication, and its role in the mitotic cell cycle may not be limited to DNA replication as cdc7 mutants effect transcriptional repression at the HMR silent mating type locus (18–20). The kinase activity of the Cdc7p in S. cerevisiae is cell cycle-regulated with activity peaking late in G1 just prior to initiation of DNA replication (21, 22). Cdc7p interacts genetically with Orc2p, the second subunit of the origin recognition complex (ORC), and with Dbf4p, also an origin targeted factor (23–26). The Dbf4p also interacts with the Polo-like protein kinase Cdc5p (26). Dbf4p is required late in G1 and plays a role in activating Cdc7p (21). We report here the identification in S. cerevisiae of a recessive loss-of-function mutant mcm5/cdc46-bob1, which bypasses the requirement for Cdc7p and its interacting factor Dbf4p (21). This result suggests that one role for Cdc7p late in G1 is to remove or bypass a Mcm5p/Cdc46p dependent block to the initiation of DNA replication.
MATERIALS AND METHODS
General Methods.
The yeast strains were grown in yeast extract/peptone/dextrose with 2% glucose or in synthetic minimal media supplemented with appropriate amino acids and glucose. 5-Fluoro-orotic acid plates were made according to Boeke et al. (27). Yeast transformations were by the lithium acetate method, and general genetic manipulations were conducted as described previously (21, 24). YCp50-CDC46 (pRB541) contains a 15.6-kb fragment containing the wild-type CDC46 gene (7). pRS306-CDC46 (pCH781) was constructed by cloning the 1.1-kb BamHI CDC46 fragment from pRB541 into pRS306. Targeted integration was completed by linearizing pCH781 with SnaBI prior to transforming diploid strain YM1: dbf4-1/dbf4-1 cdc46-bob1/CDC46, to create a cdc46::URA3 disruption. pCH802 was constructed by cloning the 5.5-kb CDC46 fragment from pRB541 into pRS414. pCH813 was constructed by ligation of two CDC46 fragments derived from pCH802, a 2.5 kb SalI–SnaBI, and a 800-bp SnaBI–SacI into SalI–SmaI cut pRS414. pCH804 was generated by gap repair of pCH802 digested with MluI/AflII and transformed into strain P194 (dbf4-1 bob1-1). pCH804 was isolated from one of the temperature-resistant colonies. pCH824 contains the 3.3-kb SalI–SnaBI/SnaBI–SacI fragment of cdc46-bob1 derived from pCH804 and cloned into pRS414. pCH819 contains the SalI–SnaBI wild-type CDC46 fragment from pCH802 and the 800-bp SnaBI–SacI cdc46-bob1 mutant fragment from pCH804 cloned into pRS414. pCH823 contains the 2.5-kb SalI–SnaBI cdc46-bob1 mutant gene fragment from pCH802 and the 800-bp SnaBI–SacI wild-type CDC46 gene fragment from pCH804 cloned into pRS414. pCH822 contains the 1.6-kb SalI–BamHI cdc46-bob1 mutant gene fragment from pCH802 and the 900-bp BamHI–SnaBI and 800-bp SnaBI–SacI wild-type CDC46 gene fragments from pCH804 cloned into pRS414. The SalI–BamHI fragment in pcdc46-bob was sequenced with Sequenase (United States Biochemical) on double-stranded templates. The sequence was determined on both strands using synthetic oligonucleotide primers.
Strains.
All the strains used in this report are isogenic with A364a (28). The presence of the bob1 allele in segregants was followed by the suppression of the cdc7 temperature-sensitive phenotype using diploid strains that have a homozygous cdc7-1/cdc7-1 genotype (21, 29). The cdc7-1, cdc46-1, and cdc42-1 temperature-sensitive mutations were followed by complementation tests. The following MATa/MATα diploid strains were used: P251/653 cdc7/cdc7 bob1/BOB1 URA4/ura4; P251/696 cdc7/cdc7 bob1/BOB1 RCK2/rck2::TRP1; 699 cdc46–1/CDC46 RCK2/rck2::TRP1; 660/694 cdc42/CDC42 RCK2/rck2::TRP1; 670 cdc7/cdc7 cdc42/CDC42 BOB1/bob1; 701 cdc7/cdc7 bob1/cdc46-1.
Fluorescence-Activated Cell Sorter (FACS) Analysis.
Cells of yeast strains 421 BOB1 CDC7; P180 bob1 CDC7; P208 BOB1 cdc7-4; P171 cdc7-4 bob1; P190 cdc7::HIS3 bob1 are isogenic with A364a, grown in rich media at 22°C and processed for FACS analysis as described (30). Yeast strains 311 MATa bar1-1 trp1-289 his6 leu2-3,112 ura3-52 can1 and 728 MATa bar1-1 trp1-289 his6 leu2-3,112 ura3-52 lys2 his3Δ1 cdc46-bob1 were used in the FACS analysis reported in Fig. 3. Cell numbers and sizes were determined using a Coulter Multisizer II using an aperture tube with a 100-μm orifice and latex beads as size standards.
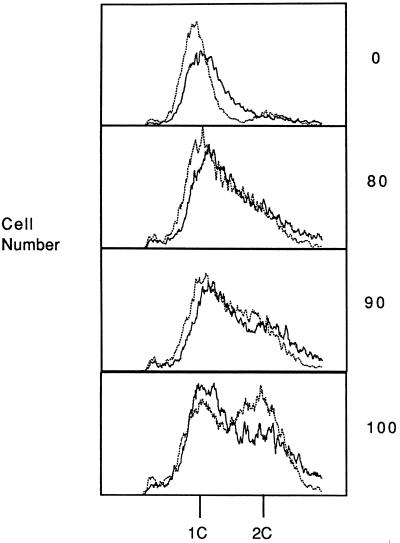
Figure 3.
FACS analysis of wild-type and cdc46-bob1 strains. Cells of wild-type strain 311 (dark solid line) and cdc46-bob1 strain 728 (light dotted line) were synchronized by α-factor treatment at 22°C in rich medium and samples were analyzed by FACS as described (30). Cell number is plotted versus the DNA content of the cells. Depicted are samples taken at 0, 80, 90, and 100 min after release from α-factor. 1C and 2C indicates the position of G1 and G2 phase cells, respectively. Yeast strains 311 and 728 are isogenic with A364a.
RESULTS
bob1 Suppression Is Specific to dbf4-1 and cdc7-1.
To examine the role for Cdc7p late in G1, the bob1 mutation has been further characterized. The bob1 mutation is a suppressor of both cdc7 and dbf4 null mutations, but it does not suppress mutations in other genes that act during G1 including cdc28-1 and cdc4-1 (21). We now report that bob1 does not bypass the requirement for a number of other essential G1/S (orc2-1, cdc6-1), S (cdc2-, cdc8-, cdc17-1), G2 (cdc13-1), or M (cdc14-1, cdc15-2, cdc16-1) phase functions (31, 32).
BOB1 Is MCM5/CDC46.
Previous studies suggested that BOB1 might encode a factor required for initiation of DNA replication. Therefore one strategy we took to identify BOB1 was to transform the bob1 dbf4 strain with plasmids harboring genes thought to play a role in initiation of DNA replication. We found that a plasmid expressing MCM5/CDC46 (pCDC46) complemented the bob1 mutant phenotype (giving suppression of the dbf4-1 temperature-sensitive defect, see Table 1). The bob1 mutation was coincidentally mapped to the right arm of chromosome XII ≈5 centimorgans (cM) from RCK2 (Table 2). This correlated with the genetic and physical maps of chromosome XII which place CDC46 2.8 cM and 60 kb from RCK2, respectively (Table 2).
Table 1.
Complementation of bob1 by MCM5/CDC46
| Strains | Plasmid | 23°C | 37°C |
|---|---|---|---|
| cdc7-1 BOB1 | + | − | |
| dbf4-1 BOB1 | + | − | |
| cdc7-1 bob1 | Vector | + | + |
| dbf4-1 bob1 | Vector | + | + |
| cdc7-1 bob1 | pCDC46 | + | − |
| dbf4-1 bob1 | pCDC46 | + | − |
| cdc7-1/cdc7-1 bob1/BOB1 | + | − | |
| dbf4-1/dbf4-1 bob1/BOB1 | + | − | |
| dbf4-1/dbf4-1 bob1/cdc46::URA3 | + | + | |
| dbf4-1 cdc46::URA3 | pCDC46 | + | − |
| dbf4-1 cdc46::URA3 | pcdc46-bob1 | + | + |
| cdc7-1/cdc7-1 cdc46-1/bob1 | + | + | |
| cdc-46-1 cdc7::HIS3 | pRS316CDC7 | +* | − |
| cdc46-1 cdc7::HIS3 | pRS316CDC7 | −† | − |
The + indicates growth and the − indicates no growth after 5 days on rich or selective media. Vector is pRS414 or pRS316.
Rich media.
The cdc46-1 cdc7∷HIS3 pRS316-CDC7 strain gave no growth on media containing 5-fluoro-orotic acid at 23°C, 27°C, 30°C, 34°C, or 37°C.
Table 2.
Genetic mapping of the BOB1 gene
| Gene pair | Ascus class
|
Distance, cM | ||
|---|---|---|---|---|
| PD | NPD | T | ||
| URA4-BOB1 | 5 | 5 | 24 | No linkage |
| RCK2-BOB1 | 26 | 0 | 3 | 5.2 |
| RCK2-CDC42 | 14 | 0 | 3 | 8.8 |
| RCK2-CDC46 | 17 | 0 | 1 | 2.8 |
| CDC42-BOB1 | 29 | 0 | 8 | 10.8 |
| CDC46-BOB1 | 40 | 0 | 0 | <1.3 |
Mapping by tetrad analysis and map distance in cM was performed as described (29). PD, NPD, and T refer to parental ditype, nonparental ditype, and tetratype asci, respectively.
If BOB1 is CDC46, then the temperature sensitivity of a diploid strain (dbf4-1/dbf4-1 bob1/BOB1) carrying a disruption in only one of the CDC46 genes will depend on which of the alleles of CDC46 is disrupted. Disruption of the wild-type CDC46 gene should suppress the dbf4-1 temperature sensitivity. In contrast, disruption of the bob1 gene should maintain the temperature-sensitive (ts) phenotype. We found that the disruption of the CDC46 loci in the diploid strain YM1 (dbf4-1/dbf4-1 bob1/BOB1) using pcdc46::URA3, created two classes of Ura+ strains, both ts and TS (nontemperature sensitive), indicating that BOB1 and CDC46 are most likely the same gene. CDC46 is an essential gene and as expected, sporulation and tetrad dissection analysis of 50 asci of both classes of Ura+ strains gave two live spores and two dead spores and the viable spores were consistently Ura−. If bob1 and CDC46 are completely linked all the spores derived from the TS and ts Ura+ strains should give spores which are TS and ts, respectively, and they consistently did. Subsequent genetic analysis confirmed tight linkage of bob1-1 and cdc46-1 to <1.3 cM (Table 2). The cdc46-bob1 gene was rescued from the chromosome using plasmid gap repair (see Materials and Methods) and the plasmid, pcdc46-bob1, complemented a cdc46::URA3 strain, and it suppressed the dbf4-1 temperature sensitivity (Table 1).
bob1 Mutation Results in a Pro → Leu Substitution at Residue 83 of Mcm5p/Cdc46p.
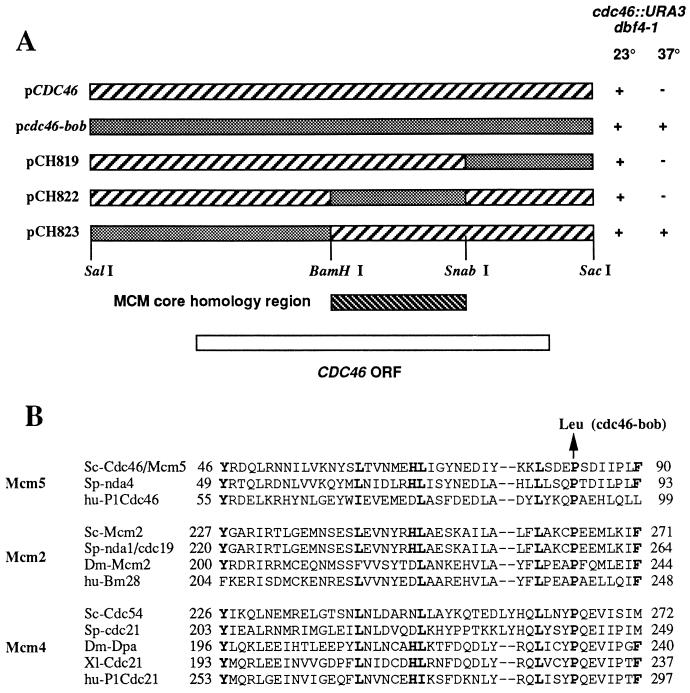
Several observations suggested that the bob1 mutation was novel. We have not been able to construct dbf4 null mutants in either mcm5/cdc46-1, mcm4/cdc54-1, or mcm7/cdc47-1 mutant strains, although they were readily obtained in a bob1 mutant strain (7). In agreement, in Table 1 the mcm5/cdc46-1 temperature-sensitive mutation does not suppress a null mutation in CDC7. To finely map the bob1 mutation, chimeras containing both wild-type and mutant CDC46 sequences were expressed in a cdc46 null (cdc46::URA3) dbf4-1 strain. As shown in Fig. 1A only Cdc46p expressed from plasmids that contain a SalI–BamHI fragment derived from the bob1 mutant gene both complement the cdc46 null and suppress the dbf4-1 temperature sensitivity. The central region in the various MCM proteins is highly conserved among MCM family members and is located between amino acids 373 and 551 in Cdc46p (8). As shown in Fig. 1A, the bob1 mutation is outside the MCM conserved core region.
Figure 1.
The bob1 mutation results in a Pro → Leu substitution at residue 83 of Mcm5p/Cdc46p. (A) bob1::URA3 dbf4-1 strains expressing wild-type Cdc46p, mutant cdc46-bob1p, or mutant/wild-type chimeras were tested for growth at 23°C and 37°C. +, Growth; −, no growth after 5 days on rich media. (B) Pro is conserved among Mcm2p, Mcm4p, and Mcm5p family members. The conserved residues are shown in boldface type.
When the entire SalI–BamHI mutant fragment was sequenced and compared with the sequence of the wild-type fragment, we found no nucleotide changes in the upstream promotor region, but we did find seven base pair changes between the mutant and wild-type fragments in the CDC46 coding sequence. The wild-type fragment was derived from strain S288C and its sequence was identical to the yeast genome project sequence, while the mutant fragment was derived from strain A364a. Six of the seven changes were conservative and resulted in no changes in the primary amino acid sequence. The remaining change was a G to A transition mutation in the noncoding strand, which resulted in a Pro → Leu substitution at residue 83 (CCT to CTT codon change), and is therefore responsible for the suppressor phenotype of bob1. Interestingly, as shown in Fig. 1B, the region containing the cdc46-bob1 mutation is evolutionarily conserved in the Mcm2p and the Mcm4p as well as in the Mcm5p, but not in the Mcm3p, Mcm6p, and Mcm7p members of the MCM family (1, 2).
bob1 Cells May Enter S Phase Prematurely.
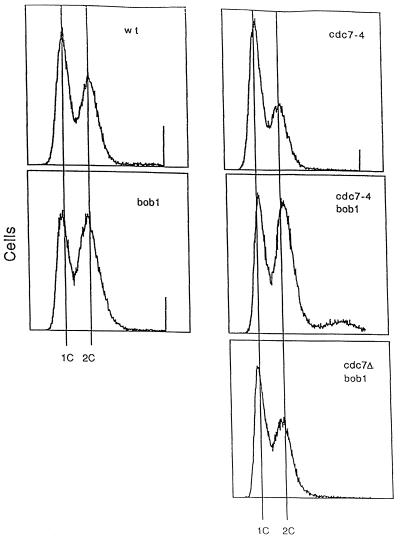
Comparison of the cell cycle distribution by FACS analysis of isogenic bob1 and wild-type strains showed that more cells from the bob1 strain displayed a 2N DNA content (Fig. 2). In contrast, the majority of cdc7-4 cells even when grown under permissive conditions, had a 1N DNA content, an effect that was more pronounced when cdc7-4 cells were grown under restrictive conditions (data not shown), while a cdc7Δ (cdc7::HIS3) bob1 strain had a nearly normal cell cycle distribution (Fig. 2). Therefore, reduction of Cdc7p activity causes more cells to accumulate in G1, whereas a bob1 mutation results in more cells finishing S phase.
Figure 2.
Cell cycle distribution of cdc7 and bob1 strains. Cell number is plotted versus the DNA content of the cells. 1C and 2C indicate the amount of DNA in a G1 and G2 phase haploid cell, respectively. Cells of yeast strains 421 BOB1 CDC7, P180 bob1 CDC7, P208 BOB1 cdc7-4, P171 cdc7-4 bob1, and P190 cdc7::HIS3 bob1 are isogenic with A364a, grown in rich media at 22°C and processed for FACS analysis as described (30).
The difference in FACS profiles between the bob1 and the wild-type cells might be explained if the bob1 cells were to enter S phase more quickly than the wild-type cells. To test this hypothesis, we used cultures synchronized with α-factor to measure the transit from G1 to G2 of the cell cycle (Fig. 3). As detected by FACS analysis the number of cells entering G2 was fairly similar for both wild-type and bob1 cultures up to 80 min after the release from α-factor. After 100 min, more cells in the bob1 mutant population than in the wild-type population were able to enter and complete S phase. We conclude that the bob1 mutation decreases, to a small extent, the time it takes to progress from G1 to G2 of the cell cycle, presumably by decreasing the time of entry into S phase.
DISCUSSION
We report here the identification in S. cerevisiae of a new allele of mcm5/cdc46 previously called bob1, which can provide the essential functions of CDC46 and yet bypass the requirement for CDC7 and DBF4. The bob1 mutant does not suppress the requirement for several other cell cycle functions and is therefore probably specific to CDC7 and DBF4. We have identified the bob1 mutation to be a single base change that converts a Pro to a Leu. This Pro residue is evolutionarily conserved in three of the MCM family members, Mcm2p, Mcm4p, and Mcmp5. This conservation in some but not all members of the MCM family suggests that there may be a functional dichotomy among the members of the MCM family. The accumulation of bob1 cells in G2 and the relative speed with which they progress from G1 to G2 as detected by FACS analysis suggest that the bob1 mutant causes an early or slightly premature entry into S phase. Such a premature entry might simply be due to the lack of the requirement for the Cdc7p step in the bob1 cells.
Cells with a temperature-sensitive mutation in CDC7 arrest late in G1 with the MCM proteins localized in the nucleus and with preinitiation complexes bound at origins (5, 33). MCM5/CDC46 executes its function early in S phase, just upstream of the execution point of hydroxyurea (12). The results presented here are therefore consistent with a role for Cdc7p as a modifier of Cdc46p/Mcm5p late in G1/early in S. This modification might promote the dissociation of the MCM proteins from chromatin. A more general interpretation of these results is that there is a Cdc46p function which opposes the initiation of DNA replication and makes initiation dependent on the activity of Cdc7p. In other words, the MCM proteins bound to chromatin during G1 block initiation, and Cdc7p is required to bypass or inactivate this block.
Recent studies in Xenopus indicate that the association of the MCM proteins with chromatin is dependent on chromatin bound Cdc6p/Cdc18p and ORC proteins (34–36). It is tempting to speculate that the chromatin bound MCM proteins inhibit the actions of the Cdc6p/Cdc18p and/or the ORC proteins. This inhibition may be overcome when Cdc7p phosphorylates these factors and/or the MCM proteins. Such a mechanism would be analogous to cyclin D/Cdk4 inactivation of Rb which allows E2F to dissociate from Rb and its inhibitory effects, and thus transcriptionally activate S phase-specific genes (37, 38). Such contrasting roles in DNA replication, essential yet inhibitory, are also similar to the functions of the phage λ P protein (39). A phage λ P protein-like activity for the MCM proteins has previously been proposed (40).
Acknowledgments
We thank L. Hartwell, J. Rine, D. Botstein, and P. Sunnerhagen for strains and S. Wente and K. Blumer for critical reading of the paper. This research was supported by Public Health Service Grant GM35078 to R.A.S. and by the Department of Cell Biology and a Damon Runyon/Walter Winchell Postdoctoral fellowship to C.F.J.H. (DRG-1203).
ABBREVIATIONS
- FACS
fluorescence-activated cell sorter
- Cdk
cyclin-dependent kinase
- cM
centimorgan
References
- 1.Chong J P J, Thommes P, Blow J J. Trends Biochem Sci. 1996;21:102–106. [PubMed] [Google Scholar]
- 2.Kearsey S E, Maiorano D, Holmes E C, Todorov I T. BioEssays. 1996;18:183–190. doi: 10.1002/bies.950180305. [DOI] [PubMed] [Google Scholar]
- 3.Stillman B. Science. 1996;274:1659–1664. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]
- 4.Maine G T, Sinha P, Tye B. Genetics. 1984;106:365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan H, Gibson S, Tye B K. Genes Dev. 1991;5:944–957. doi: 10.1101/gad.5.6.944. [DOI] [PubMed] [Google Scholar]
- 6.Whitbread L A, Dalton S. Gene. 1995;155:113–117. doi: 10.1016/0378-1119(94)00925-i. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy K, Lee A, Chen E, Botstein D. Genes Dev. 1991;5:958–969. doi: 10.1101/gad.5.6.958. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Hennessy K, Botstein D, Tye B. Proc Natl Acad Sci USA. 1992;89:10459–10463. doi: 10.1073/pnas.89.21.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moir D, Stewart S E, Osmond B C, Botstein D. Genetics. 1982;100:547–563. doi: 10.1093/genetics/100.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton S, Whitbread L. Proc Natl Acad Sci USA. 1995;92:2514–2518. doi: 10.1073/pnas.92.7.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong J P J, Mahbubani H M, Khoo C Y, Blow J J. Nature (London) 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- 12.Hennessy K M, Clark C D, Botstein D. Genes Dev. 1990;4:2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- 13.Kimura H, Nozaki N, Sugimoto K. EMBO J. 1994;13:4311–4320. doi: 10.1002/j.1460-2075.1994.tb06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Cell. 1995;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 15.Romanowski P, Madine M, Laskey R. Proc Natl Acad Sci USA. 1996;93:10189–10194. doi: 10.1073/pnas.93.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coue M, Kearsey S E, Mechali M. EMBO J. 1996;15:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrickson M, Madine M, Dalton S, Gautier J. Proc Natl Acad Sci USA. 1996;93:12223–12228. doi: 10.1073/pnas.93.22.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schild D, Byers B. Chromosoma. 1978;70:109–130. doi: 10.1007/BF00292220. [DOI] [PubMed] [Google Scholar]
- 19.Simchen G. Genetics. 1974;76:745–753. doi: 10.1093/genetics/76.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelrod A, Rine J. Mol Cell Biol. 1991;11:1080–1091. doi: 10.1128/mcb.11.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson A L, Pahl P M B, Harrison K, Rosamond J, Sclafani R A. Mol Cell Biol. 1993;13:2899–2908. doi: 10.1128/mcb.13.5.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon H, Loo S, Cambell J L. Mol Biol Cell. 1993;4:195–208. doi: 10.1091/mbc.4.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo S, Fox C A, Rine J, Kobayashi R, Stillman B, Bell S. Mol Biol Cell. 1995;6:741–756. doi: 10.1091/mbc.6.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy C F J. Mol Cell Biol. 1996;16:1832–1841. doi: 10.1128/mcb.16.4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowell S J, Romanowski P, Diffley J F X. Science. 1994;265:1243–1246. doi: 10.1126/science.8066465. [DOI] [PubMed] [Google Scholar]
- 26.Hardy C F J, Pautz A. Mol Cell Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boeke J D, LaCroute F, Fink G. Mol Gen Genet. 1984;197:521–525. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 28.Hereford L M, Hartwell L M. J Mol Biol. 1974;84:445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- 29.Pahl, P. M. B. (1994) Doctoral thesis (Univ. of Colorado, Denver).
- 30.Ostroff R M, Sclafani R A. Mutat Res. 1995;329:143–152. doi: 10.1016/0027-5107(95)00030-m. [DOI] [PubMed] [Google Scholar]
- 31.Pringle J R, Hartwell L H. In: The Molecular Biology of the Yeast Saccharomyces. Strathern J N, Jones E W, Broach J R, editors. Vol. 1. Plainview, NY: Cold Spring Harbor Lab. Press; 1981. pp. 97–142. [Google Scholar]
- 32.Foss M, McNally F J, Laurenson P, Rine J. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 33.Diffley J F X, Cocker J H, Dowell S J, Rowley A. Cell. 1994;78:303–316. doi: 10.1016/0092-8674(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 34.Coleman T R, Carpenter P B, Dunphy W G. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 35.Rowles A, Chong J, Brown L, Howell M, Evan G, Blow J. Cell. 1996;87:287–296. doi: 10.1016/s0092-8674(00)81346-x. [DOI] [PubMed] [Google Scholar]
- 36.Romanowski P, Madine M, Rowles A, Blow J, Laskey R A. Curr Biol. 1996;6:1416–1425. doi: 10.1016/s0960-9822(96)00746-4. [DOI] [PubMed] [Google Scholar]
- 37.Sherr C J. Trends Cell Biol. 1994;4:15–18. doi: 10.1016/0962-8924(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 39.Kornberg A, Baker T A. DNA Replication. New York: Freeman; 1991. [Google Scholar]
- 40.Stillman B. J Biol Chem. 1994;10:7047–7050. [PubMed] [Google Scholar]