Abstract
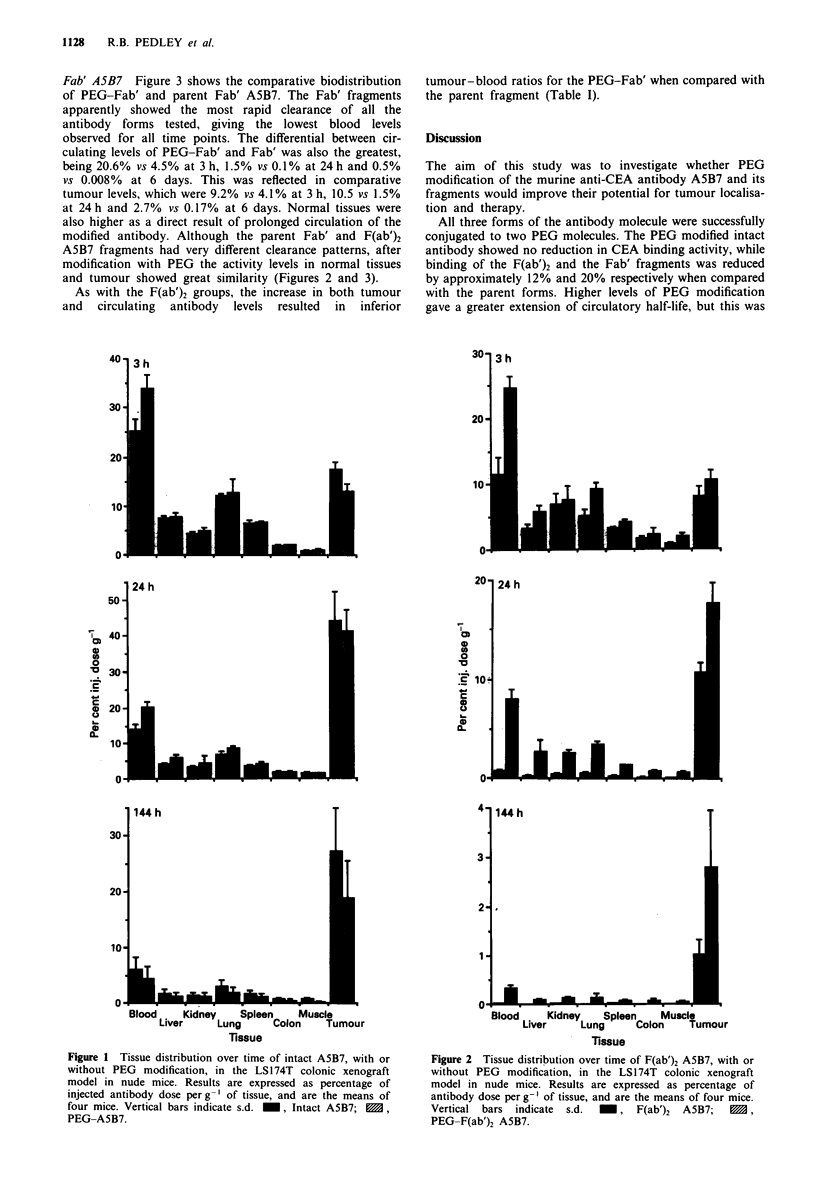
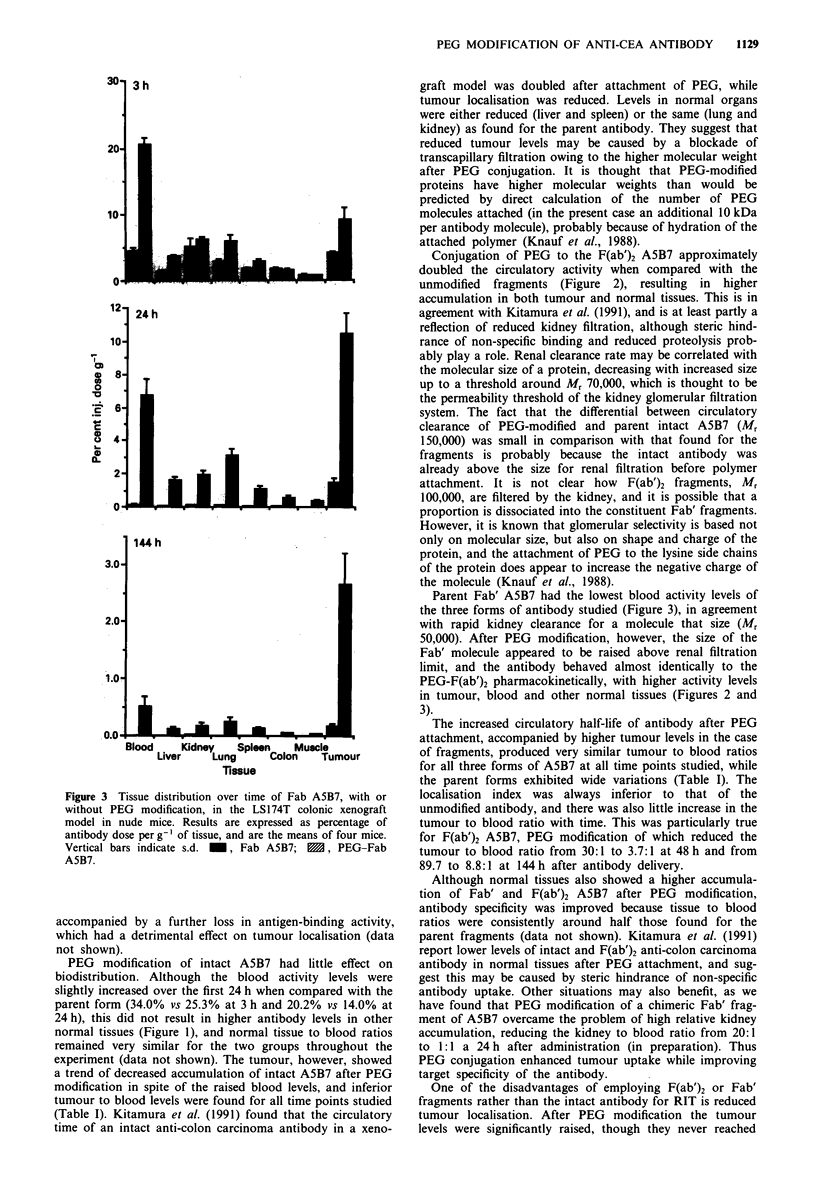
Attachment of poly(ethylene glycol) (PEG) to proteins can greatly alter their pharmacological properties, including extending the plasma half-life and reducing immunogenicity, both of which are potentially beneficial to tumour targeting. IgG, F(ab')2 and Fab' fragments of the anti-CEA antibody A5B7 were chemically modified with PEG (M(r) 5,000), labelled with 125I and their pharmacokinetics compared with the unmodified forms in the LS174T colonic xenograft in nude mice. PEG modification of the intact antibody had little effect on biodistribution, although tumour localisation was slightly reduced. In contrast, similar modification of F(ab')2 and Fab'A5B7 significantly prolonged plasma half-life and increased radioantibody accumulation in the tumour and to a lesser extent in normal tissues, but reduced tissue to blood ratios. Prior to modification, Fab' A5B7 (M(r) 50,000) cleared more rapidly from the circulation than F(ab')2 (M(r) 100,000), but after PEG attachment their biodistributions converged, while the tumour to blood ratios were reduced and resembled that of the intact antibody. The enhanced tumour accumulation, reduced normal tissue to blood ratios and potentially reduced immunogenicity of fragments after PEG attachment may therefore prove superior to either unmodified fragments or intact antibody for antibody-targeted therapy, although the increased plasma half-life may necessitate the use of a clearance mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begent R. H., Ledermann J. A., Green A. J., Bagshawe K. D., Riggs S. J., Searle F., Keep P. A., Adam T., Dale R. G., Glaser M. G. Antibody distribution and dosimetry in patients receiving radiolabelled antibody therapy for colorectal cancer. Br J Cancer. 1989 Sep;60(3):406–412. doi: 10.1038/bjc.1989.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begent R. H., Pedley R. B. Antibody targeted therapy in cancer: comparison of murine and clinical studies. Cancer Treat Rev. 1990 Sep;17(2-3):373–378. doi: 10.1016/0305-7372(90)90071-m. [DOI] [PubMed] [Google Scholar]
- Boxer G. M., Abassi A. M., Pedley R. B., Begent R. H. Localisation of monoclonal antibodies reacting with different epitopes on carcinoembryonic antigen (CEA)--implications for targeted therapy. Br J Cancer. 1994 Feb;69(2):307–314. doi: 10.1038/bjc.1994.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchegger F., Pèlegrin A., Delaloye B., Bischof-Delaloye A., Mach J. P. Iodine-131-labeled MAb F(ab')2 fragments are more efficient and less toxic than intact anti-CEA antibodies in radioimmunotherapy of large human colon carcinoma grafted in nude mice. J Nucl Med. 1990 Jun;31(6):1035–1044. [PubMed] [Google Scholar]
- Kitamura K., Takahashi T., Yamaguchi T., Noguchi A., Noguchi A., Takashina K., Tsurumi H., Inagake M., Toyokuni T., Hakomori S. Chemical engineering of the monoclonal antibody A7 by polyethylene glycol for targeting cancer chemotherapy. Cancer Res. 1991 Aug 15;51(16):4310–4315. [PubMed] [Google Scholar]
- Knauf M. J., Bell D. P., Hirtzer P., Luo Z. P., Young J. D., Katre N. V. Relationship of effective molecular size to systemic clearance in rats of recombinant interleukin-2 chemically modified with water-soluble polymers. J Biol Chem. 1988 Oct 15;263(29):15064–15070. [PubMed] [Google Scholar]
- Ledermann J. A., Begent R. H., Bagshawe K. D., Riggs S. J., Searle F., Glaser M. G., Green A. J., Dale R. G. Repeated antitumour antibody therapy in man with suppression of the host response by cyclosporin A. Br J Cancer. 1988 Nov;58(5):654–657. doi: 10.1038/bjc.1988.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D., Pedley R. B., Boden J. A., Boden R., Begent R. H. Clearance of circulating radio-antibodies using streptavidin or second antibodies in a xenograft model. Br J Cancer. 1994 Mar;69(3):502–507. doi: 10.1038/bjc.1994.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley R. B., Begent R. H., Boden J. A., Boden R., Adam T., Bagshawe K. D. The effect of radiosensitizers on radio-immunotherapy, using 131I-labelled anti-CEA antibodies in a human colonic xenograft model. Int J Cancer. 1991 Feb 20;47(4):597–602. doi: 10.1002/ijc.2910470420. [DOI] [PubMed] [Google Scholar]
- Pedley R. B., Boden J. A., Boden R., Dale R., Begent R. H. Comparative radioimmunotherapy using intact or F(ab')2 fragments of 131I anti-CEA antibody in a colonic xenograft model. Br J Cancer. 1993 Jul;68(1):69–73. doi: 10.1038/bjc.1993.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley R. B., Boden J., Keep P. A., Harwood P. J., Green A. J., Rogers G. T. Relationship between tumour size and uptake of radiolabelled anti-CEA in a colon tumour xenograft. Eur J Nucl Med. 1987;13(4):197–202. doi: 10.1007/BF00256491. [DOI] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Turner A., King D. J., Farnsworth A. P., Rhind S. K., Pedley R. B., Boden J., Boden R., Millican T. A., Millar K., Boyce B. Comparative biodistributions of indium-111-labelled macrocycle chimeric B72.3 antibody conjugates in tumour-bearing mice. Br J Cancer. 1994 Jul;70(1):35–41. doi: 10.1038/bjc.1994.246. [DOI] [PMC free article] [PubMed] [Google Scholar]



