Abstract
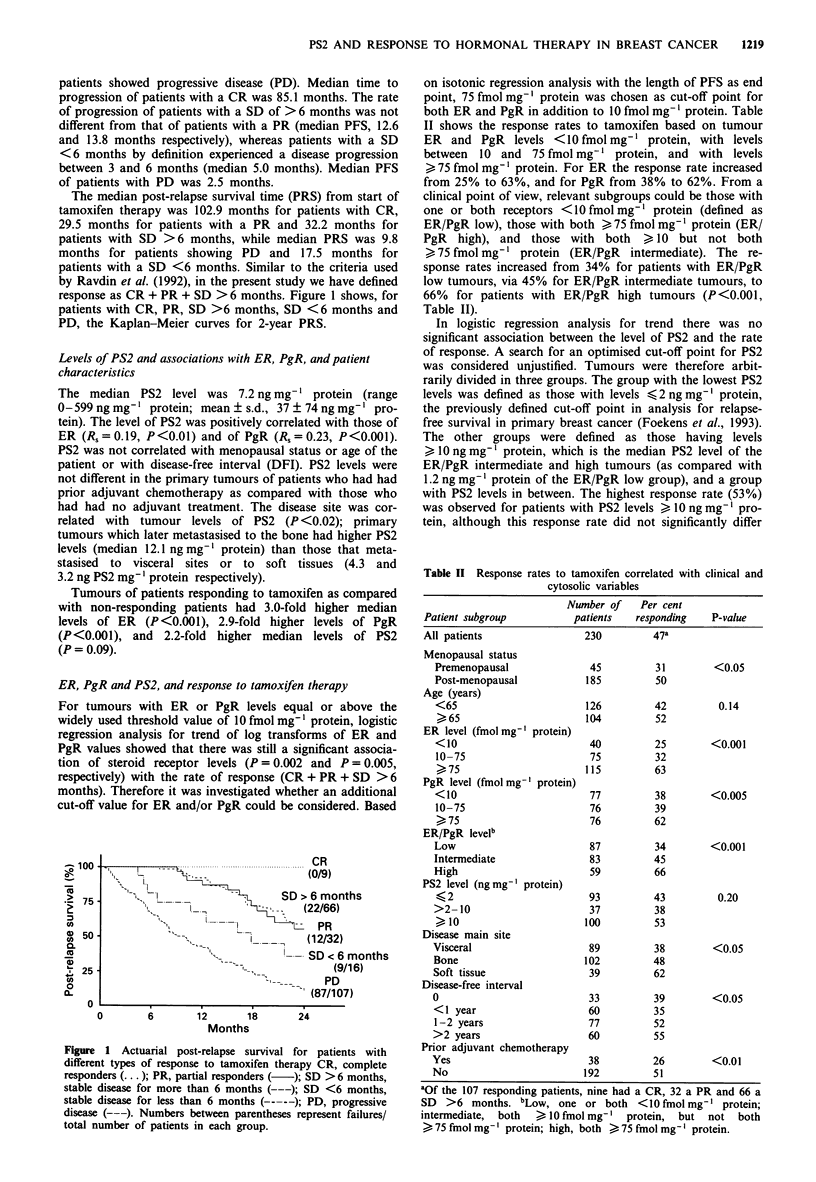
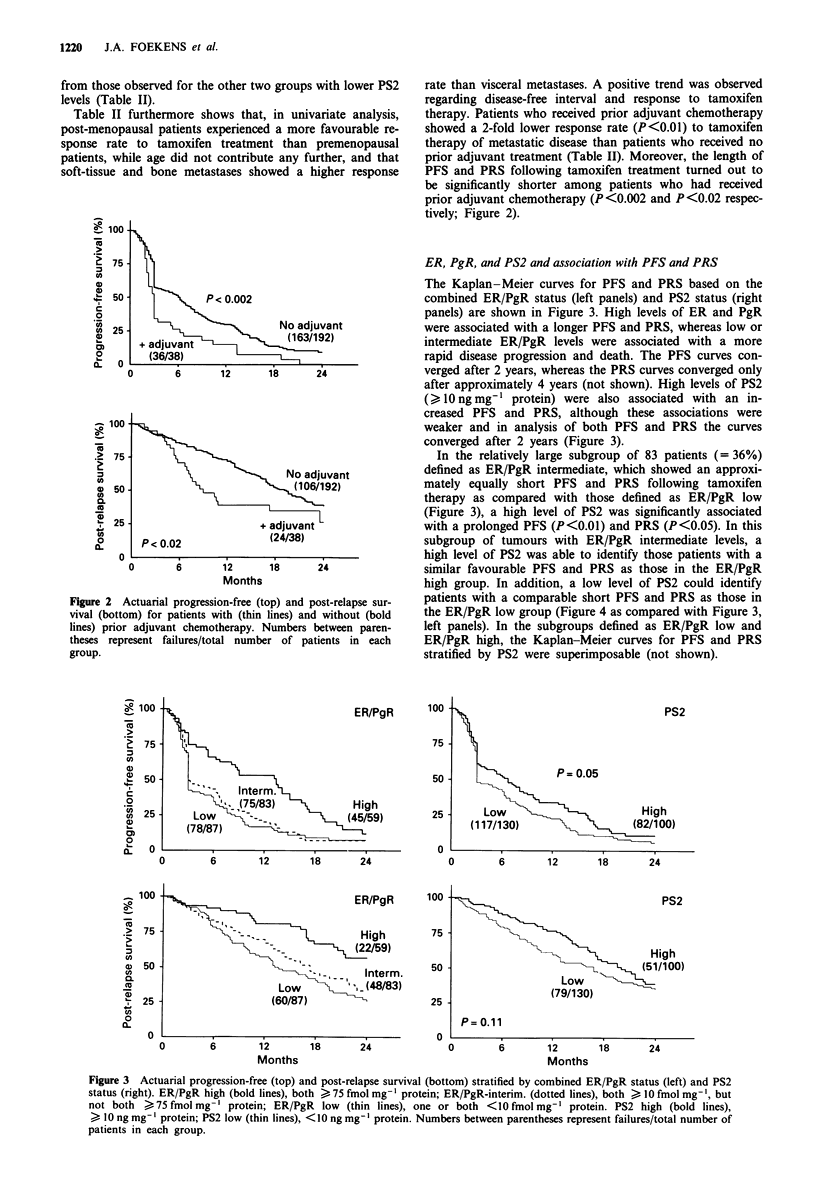
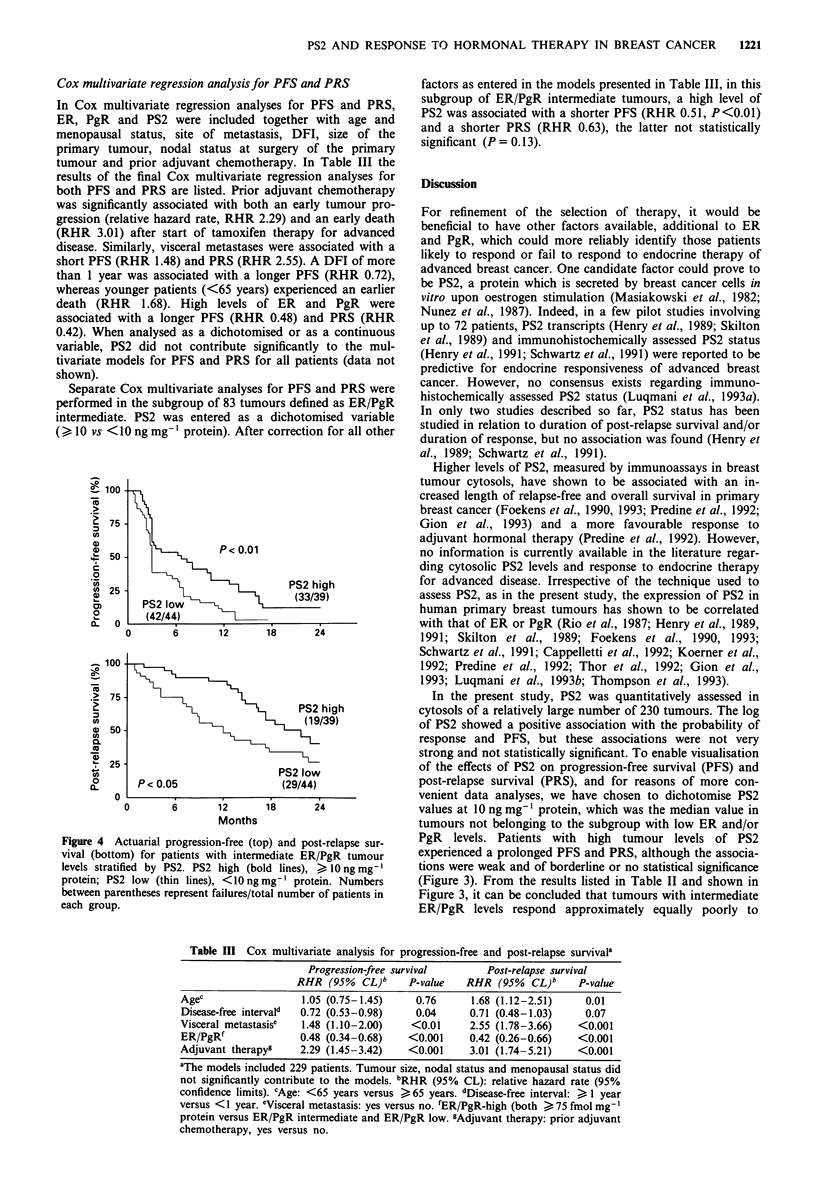
PS2, an oestrogen-inducible protein, was measured in the cytosol of 230 primary tumours from patients who were subjected to first-line tamoxifen therapy for advanced disease without prior adjuvant therapy with tamoxifen. PS2 correlated positively with oestrogen receptor (ER, P < 0.01) and progesterone receptor content (PgR, P < 0.001), and with the length of progression-free survival (PFS, P = 0.05). Although not statistically significant, higher levels of PS2 (> or = 10 ng mg-1 protein) were also associated with increased probability of response to tamoxifen treatment and a longer total post-relapse survival (PRS). ER, PgR, menopausal status, site of disease and prior adjuvant chemotherapy were all associated with response to tamoxifen therapy and with PFS. In multivariate analysis for PFS, low levels of ER and PgR, visceral metastasis, a disease-free interval of less than 1 year and prior adjuvant chemotherapy were all significantly associated with an increased probability of a rapid disease progression after start of tamoxifen therapy. In the subset of 83 tumours with intermediate levels of ER and PgR (both > or = 10, but not both > or = 75 fmol mg-1 protein), PS2 was positively related with the length of PFS (P < 0.01) and PRS (P < 0.05). PS2 remained the strongest factor in multivariate analysis for PFS (P < 0.01) in this ER/PgR intermediate subgroup, but was not of predictive value in univariate or multivariate analysis for both PFS and PRS in tumours classified as ER/PgR low or high (> or = 75 fmol mg-1 protein). It is concluded that PS2 status may be used as a parameter, additional to ER and PgR, for better refinement of prediction of response to tamoxifen treatment in advanced breast cancer patients especially with intermediate ER/PgR levels in their primary tumour.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappelletti V., Coradini D., Scanziani E., Benini E., Silvestrini R., Di Fronzo G. Prognostic relevance of pS2 status in association with steroid receptor status and proliferative activity in node-negative breast cancer. Eur J Cancer. 1992;28A(8-9):1315–1318. doi: 10.1016/0959-8049(92)90507-x. [DOI] [PubMed] [Google Scholar]
- Foekens J. A., Portengen H., van Putten W. L., Peters H. A., Krijnen H. L., Alexieva-Figusch J., Klijn J. G. Prognostic value of estrogen and progesterone receptors measured by enzyme immunoassays in human breast tumor cytosols. Cancer Res. 1989 Nov 1;49(21):5823–5828. [PubMed] [Google Scholar]
- Foekens J. A., Rio M. C., Seguin P., van Putten W. L., Fauque J., Nap M., Klijn J. G., Chambon P. Prediction of relapse and survival in breast cancer patients by pS2 protein status. Cancer Res. 1990 Jul 1;50(13):3832–3837. [PubMed] [Google Scholar]
- Foekens J. A., van Putten W. L., Portengen H., de Koning H. Y., Thirion B., Alexieva-Figusch J., Klijn J. G. Prognostic value of PS2 and cathepsin D in 710 human primary breast tumors: multivariate analysis. J Clin Oncol. 1993 May;11(5):899–908. doi: 10.1200/JCO.1993.11.5.899. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Allred D. C., Elledge R. M., Krieg S. L., Benedix M. G., Nawaz Z., O'Malley B. W., Greene G. L., McGuire W. L. The ER-positive/PgR-negative breast cancer phenotype is not associated with mutations within the DNA binding domain. Breast Cancer Res Treat. 1993;26(2):191–202. doi: 10.1007/BF00689692. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Chamness G. C., McGuire W. L. Estrogen receptor mutations in breast cancer. J Cell Biochem. 1993 Feb;51(2):135–139. doi: 10.1002/jcb.240510204. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., Allred D. C., Elledge R. M., Nawaz Z., McDonnell D. P., O'Malley B. W., Greene G. L., McGuire W. L. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992 Jan 15;52(2):483–486. [PubMed] [Google Scholar]
- Gasparini G., Pozza F., Harris A. L. Evaluating the potential usefulness of new prognostic and predictive indicators in node-negative breast cancer patients. J Natl Cancer Inst. 1993 Aug 4;85(15):1206–1219. doi: 10.1093/jnci/85.15.1206. [DOI] [PubMed] [Google Scholar]
- Gion M., Mione R., Pappagallo G. L., Gatti C., Nascimben O., Bari M., Leon A. E., Vinante O., Bruscagnin G. PS2 in breast cancer--alternative or complementary tool to steroid receptor status? Evaluation of 446 cases. Br J Cancer. 1993 Aug;68(2):374–379. doi: 10.1038/bjc.1993.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Hennessy C., Lennard T. W., May F. E., Westley B. R. Expression of the oestrogen regulated pNR-2 mRNA in human breast cancer: relation to oestrogen receptor mRNA levels and response to tamoxifen therapy. Br J Cancer. 1990 Jan;61(1):32–38. doi: 10.1038/bjc.1990.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Piggott N. H., Mallick U. K., Nicholson S., Farndon J. R., Westley B. R., May F. E. pNR-2/pS2 immunohistochemical staining in breast cancer: correlation with prognostic factors and endocrine response. Br J Cancer. 1991 Apr;63(4):615–622. doi: 10.1038/bjc.1991.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Predicting response to endocrine therapy in human breast cancer: a hypothesis. Science. 1975 Aug 29;189(4204):726–727. doi: 10.1126/science.168640. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. Mechanisms of hormone resistance in breast cancer. Breast Cancer Res Treat. 1993;26(2):119–130. doi: 10.1007/BF00689685. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Wei L. L., Sedlacek S. M., d'Arville C. N. Progestin action and progesterone receptor structure in human breast cancer: a review. Recent Prog Horm Res. 1985;41:249–316. doi: 10.1016/b978-0-12-571141-8.50010-x. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., Berns E. M., Bontenbal M., Foekens J. Cell biological factors associated with the response of breast cancer to systemic treatment. Cancer Treat Rev. 1993 Apr;19 (Suppl B):45–63. doi: 10.1016/0305-7372(93)90007-e. [DOI] [PubMed] [Google Scholar]
- Koerner F. C., Goldberg D. E., Edgerton S. M., Schwartz L. H. pS2 protein and steroid hormone receptors in invasive breast carcinomas. Int J Cancer. 1992 Sep 9;52(2):183–188. doi: 10.1002/ijc.2910520205. [DOI] [PubMed] [Google Scholar]
- Luqmani Y. A., Campbell T., Soomro S., Shousha S., Rio M. C., Coombes R. C. Immunohistochemical localisation of pS2 protein in ductal carcinoma in situ and benign lesions of the breast. Br J Cancer. 1993 Apr;67(4):749–753. doi: 10.1038/bjc.1993.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani Y. A., Ricketts D., Ryall G., Turnbull L., Law M., Coombes R. C. Prediction of response to endocrine therapy in breast cancer using immunocytochemical assays for pS2, oestrogen receptor and progesterone receptor. Int J Cancer. 1993 Jun 19;54(4):619–623. doi: 10.1002/ijc.2910540416. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire W. L., Clark G. M. Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N Engl J Med. 1992 Jun 25;326(26):1756–1761. doi: 10.1056/NEJM199206253262607. [DOI] [PubMed] [Google Scholar]
- Nunez A. M., Jakowlev S., Briand J. P., Gaire M., Krust A., Rio M. C., Chambon P. Characterization of the estrogen-induced pS2 protein secreted by the human breast cancer cell line MCF-7. Endocrinology. 1987 Nov;121(5):1759–1765. doi: 10.1210/endo-121-5-1759. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Yochmowitz M. G., Knight W. A., 3rd, McGuire W. L. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Predine J., Spyratos F., Prud'homme J. F., Andrieu C., Hacene K., Brunet M., Pallud C., Milgrom E. Enzyme-linked immunosorbent assay of pS2 in breast cancers, benign tumors, and normal breast tissues. Correlation with prognosis and adjuvant hormone therapy. Cancer. 1992 Apr 15;69(8):2116–2123. doi: 10.1002/1097-0142(19920415)69:8<2116::aid-cncr2820690818>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Green S., Dorr T. M., McGuire W. L., Fabian C., Pugh R. P., Carter R. D., Rivkin S. E., Borst J. R., Belt R. J. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992 Aug;10(8):1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens R. D., Bajetta E., Bonneterre J., Klijn J. G., Lønning P. E., Paridaens R. Treatment of relapse of breast cancer after adjuvant systemic therapy--review and guidelines for future research. Eur J Cancer. 1994;30A(1):106–111. doi: 10.1016/s0959-8049(05)80029-2. [DOI] [PubMed] [Google Scholar]
- Rubens R. D. Effect of adjuvant systemic therapy on response to treatment after relapse. Cancer Treat Rev. 1993 Apr;19 (Suppl B):3–10. doi: 10.1016/0305-7372(93)90002-9. [DOI] [PubMed] [Google Scholar]
- Schwartz L. H., Koerner F. C., Edgerton S. M., Sawicka J. M., Rio M. C., Bellocq J. P., Chambon P., Thor A. D. pS2 expression and response to hormonal therapy in patients with advanced breast cancer. Cancer Res. 1991 Jan 15;51(2):624–628. [PubMed] [Google Scholar]
- Scott G. K., Kushner P., Vigne J. L., Benz C. C. Truncated forms of DNA-binding estrogen receptors in human breast cancer. J Clin Invest. 1991 Aug;88(2):700–706. doi: 10.1172/JCI115356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilton R. A., Luqmani Y. A., McClelland R. A., Coombes R. C. Characterisation of a messenger RNA selectively expressed in human breast cancer. Br J Cancer. 1989 Aug;60(2):168–175. doi: 10.1038/bjc.1989.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyser M., Mester J. Oncogenes homologous to steroid receptors? Nature. 1985 Jun 13;315(6020):546–546. doi: 10.1038/315546a0. [DOI] [PubMed] [Google Scholar]
- Thompson A. M., Hawkins R. A., Elton R. A., Steel C. M., Chetty U., Carter D. C. pS2 is an independent factor of good prognosis in primary breast cancer. Br J Cancer. 1993 Jul;68(1):93–96. doi: 10.1038/bjc.1993.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor A. D., Koerner F. C., Edgerton S. M., Wood W. C., Stracher M. A., Schwartz L. H. pS2 expression in primary breast carcinomas: relationship to clinical and histological features and survival. Breast Cancer Res Treat. 1992;21(2):111–119. doi: 10.1007/BF01836957. [DOI] [PubMed] [Google Scholar]
- de Takats P. G., Dunn J. A., Kerr D. J., Morrison J. M. Impact of adjuvant chemotherapy in breast cancer on response to tamoxifen at relapse. Cancer Treat Rev. 1993 Apr;19 (Suppl B):11–19. doi: 10.1016/0305-7372(93)90003-a. [DOI] [PubMed] [Google Scholar]


