Abstract
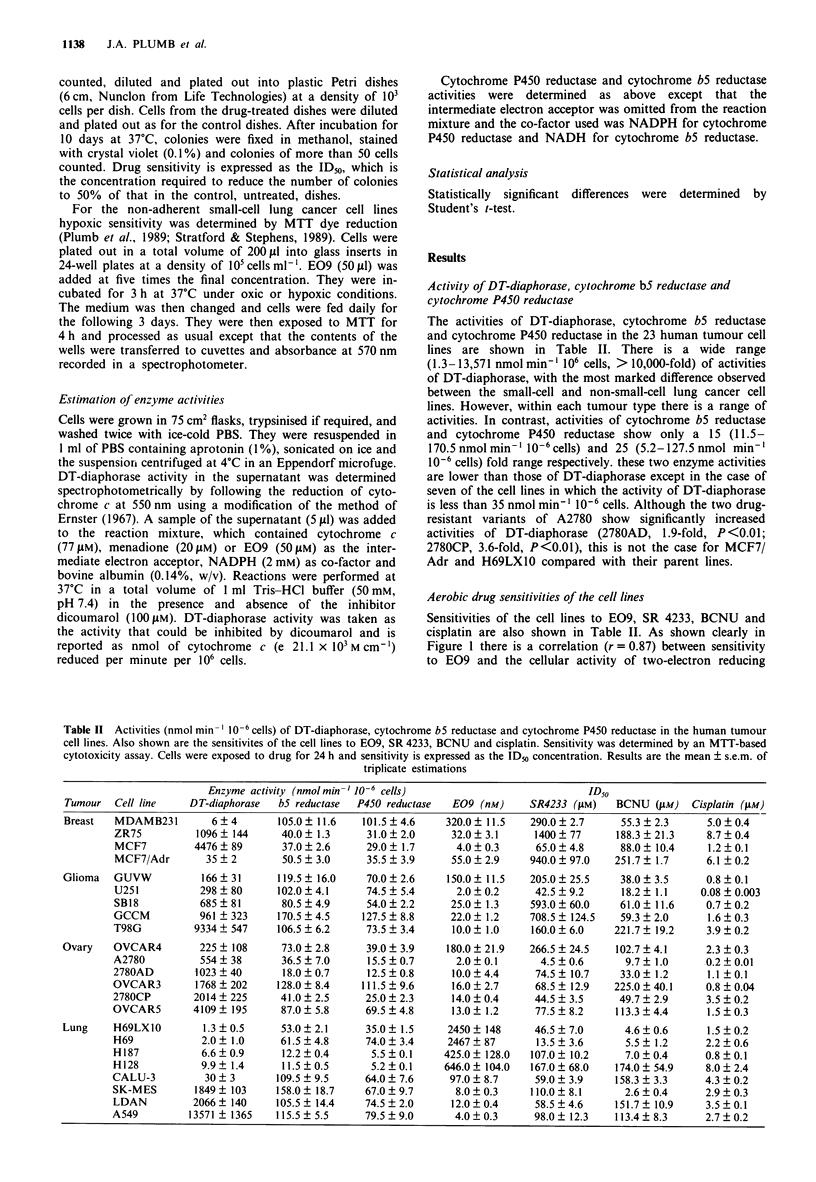
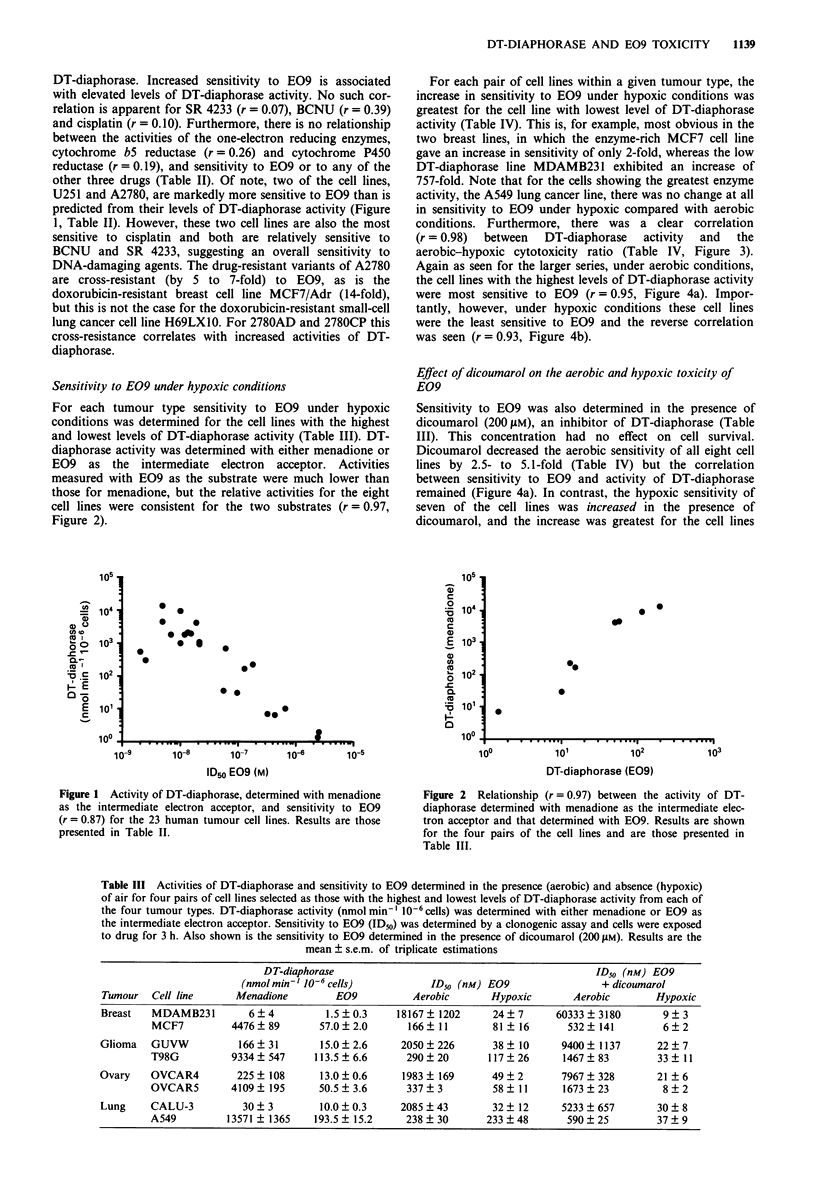
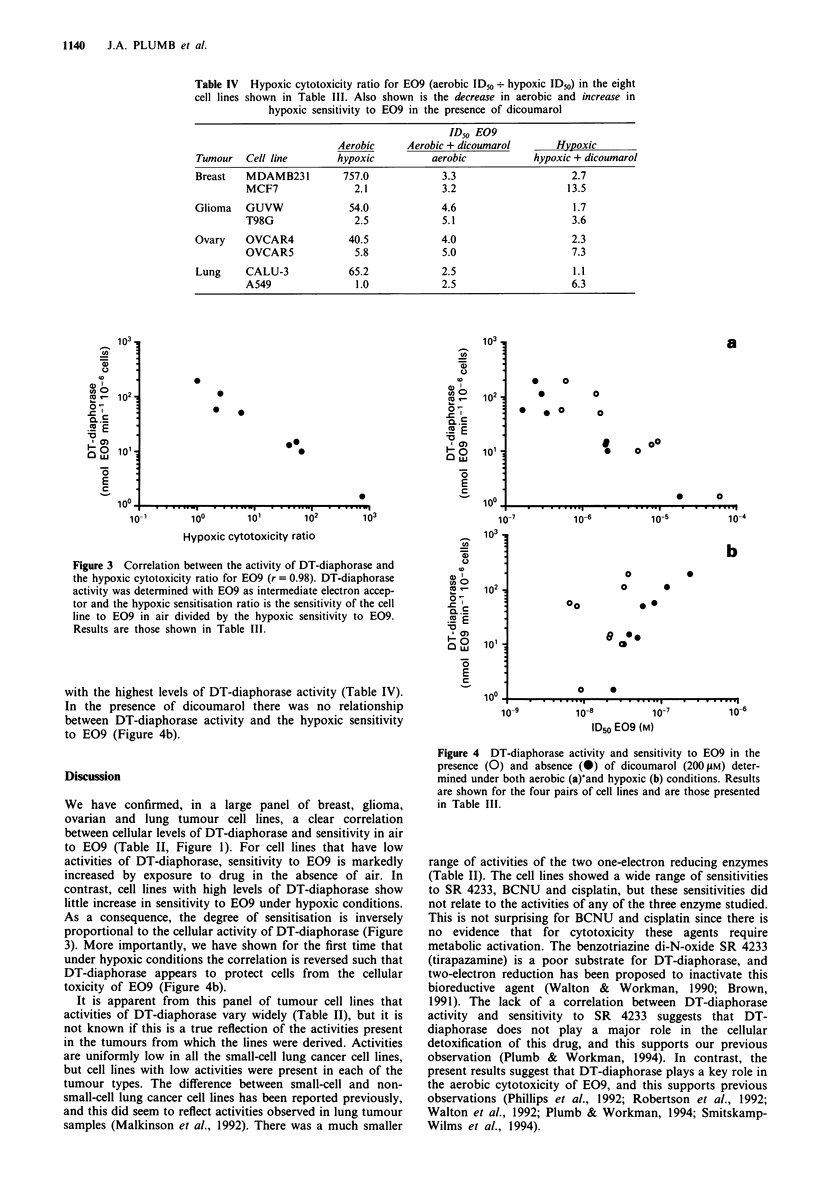
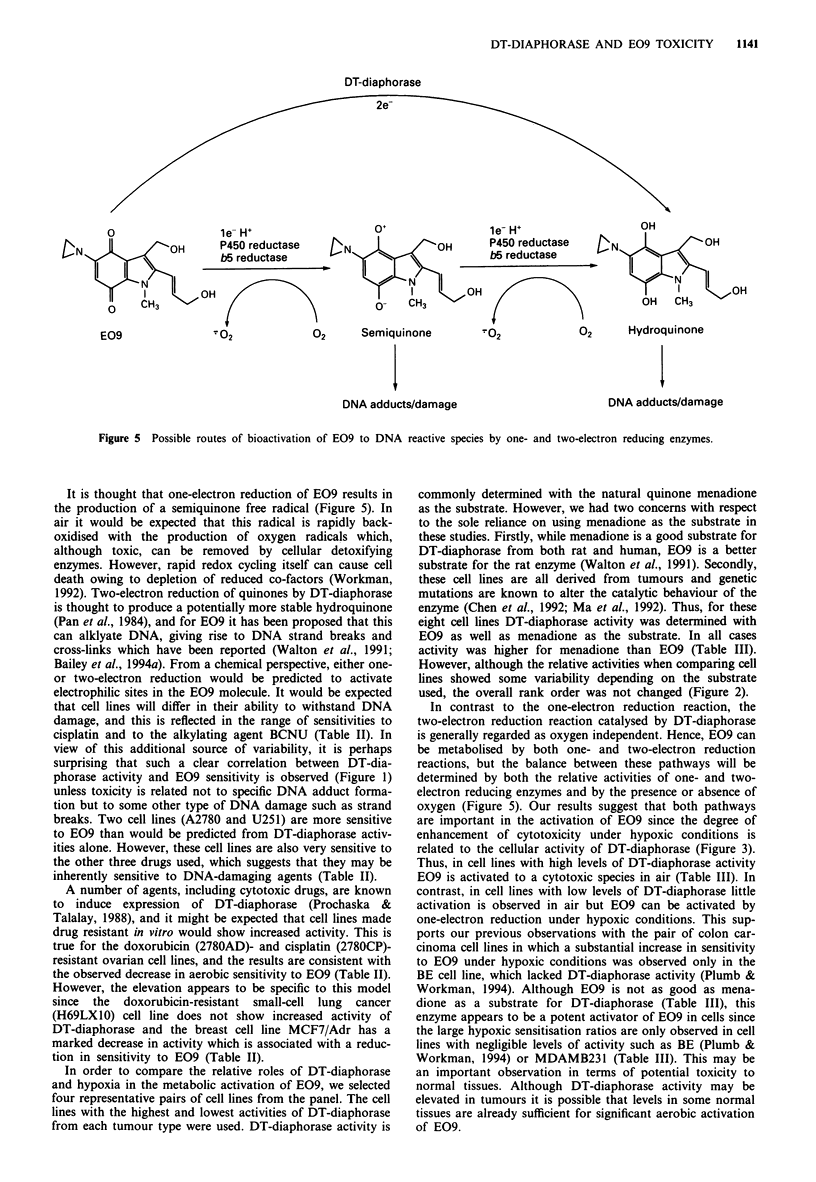
Aerobic sensitivity to indoloquinone EO9 has been shown to correlate with cellular levels of the two-electron reducing enzyme DT-diaphorase. However, little is known about the relative roles of one- and two-electron reducing enzymes in the hypoxic cytotoxicity of EO9. We have characterised a panel of 23 human tumour cell lines for both bioreductive enzyme activities and aerobic sensitivity to EO9. Eight cell lines were then selected for a comparison of aerobic and hypoxic sensitivities. Activities of DT-diaphorase showed a wide range (> 10,000-fold), while activities of the one-electron reducing cytochrome b5 and cytochrome P450 reductases were generally lower and showed only a 15- and 25-fold range respectively. The aerobic cytotoxicity of EO9 was clearly related to the cellular levels of DT-diaphorase (r = 0.87), with higher levels giving increased sensitivity, but not to the levels of one-electron reducing enzymes. In contrast, there was no relationship between sensitivity to BCNU, cisplatin or the bioreductive agent SR 4233 (tirapazamine) and activities of any of these reducing enzymes. Under hypoxic conditions sensitivity to EO9 was markedly increased in cell lines with low levels of DT-diaphorase activity, while cell lines with high levels show only a small increase in sensitivity. This is reflected by a clear correlation (r = 0.98) between cellular DT-diaphorase activity and the ratio of aerobic to hypoxic sensitivity to EO9. However, we have now for the first time demonstrated an inverse correlation (r = 0.93) between the cellular activity of DT-diaphorase and hypoxic sensitivity to EO9, that is sensitivity decreases with increasing DT-diaphorase activity. Moreover, this correlation was lost when cells were exposed to drug in the presence of dicoumarol, supporting an involvement of DT-diaphorase in this relationship. These observations question the previously straightforward role for DT-diaphorase in the metabolic activation of EO9. Whereas DT-diaphorase is associated with increased toxicity in air, it appears to reduce the cytotoxicity of EO9 in hypoxic conditions. This suggests either that the one-electron reduction product of EO9 metabolism, the semiquinone, is more toxic than the two-electron reduction product, the hydroquinone, or that the hydroquinone is not cytotoxic and aerobic toxicity is due to the transient appearance of the semiquinone upon back oxidation of the hydroquinone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bizanek R., Chowdary D., Arai H., Kasai M., Hughes C. S., Sartorelli A. C., Rockwell S., Tomasz M. Adducts of mitomycin C and DNA in EMT6 mouse mammary tumor cells: effects of hypoxia and dicumarol on adduct patterns. Cancer Res. 1993 Nov 1;53(21):5127–5134. [PubMed] [Google Scholar]
- Chen H. H., Ma J. X., Forrest G. L., Deng P. S., Martino P. A., Lee T. D., Chen S. Expression of rat liver NAD(P)H:quinone-acceptor oxidoreductase in Escherichia coli and mutagenesis in vitro at Arg-177. Biochem J. 1992 Jun 15;284(Pt 3):855–860. doi: 10.1042/bj2840855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Dulhanty A. M., Whitmore G. F. Chinese hamster ovary cell lines resistant to mitomycin C under aerobic but not hypoxic conditions are deficient in DT-diaphorase. Cancer Res. 1991 Apr 1;51(7):1860–1865. [PubMed] [Google Scholar]
- Gatenby R. A., Kessler H. B., Rosenblum J. S., Coia L. R., Moldofsky P. J., Hartz W. H., Broder G. J. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988 May;14(5):831–838. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Gustafson D. L., Pritsos C. A. Bioactivation of mitomycin C by xanthine dehydrogenase from EMT6 mouse mammary carcinoma tumors. J Natl Cancer Inst. 1992 Aug 5;84(15):1180–1185. doi: 10.1093/jnci/84.15.1180. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Pizao P. E., Berger D. P., Kooistra K. L., Bibby M. C., Boven E., Dreef-van der Meulen H. C., Henrar R. E., Fiebig H. H., Double J. A. EO9: a novel bioreductive alkylating indoloquinone with preferential solid tumour activity and lack of bone marrow toxicity in preclinical models. Eur J Cancer. 1993;29A(6):897–906. doi: 10.1016/s0959-8049(05)80434-4. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Rockwell S., Sartorelli A. C. Enhancement of mitomycin C cytotoxicity to hypoxic tumor cells by dicoumarol in vivo and in vitro. Cancer Res. 1985 Jan;45(1):213–216. [PubMed] [Google Scholar]
- Komiyama T., Kikuchi T., Sugiura Y. Generation of hydroxyl radical by anticancer quinone drugs, carbazilquinone, mitomycin C, aclacinomycin A and adriamycin, in the presence of NADPH-cytochrome P-450 reductase. Biochem Pharmacol. 1982 Nov 15;31(22):3651–3656. doi: 10.1016/0006-2952(82)90590-1. [DOI] [PubMed] [Google Scholar]
- Ma Q., Cui K., Wang R. W., Lu A. Y., Yang C. S. Site-directed mutagenesis of rat liver NAD(P)H: quinone oxidoreductase: roles of lysine 76 and cysteine 179. Arch Biochem Biophys. 1992 May 1;294(2):434–439. doi: 10.1016/0003-9861(92)90708-5. [DOI] [PubMed] [Google Scholar]
- Malkinson A. M., Siegel D., Forrest G. L., Gazdar A. F., Oie H. K., Chan D. C., Bunn P. A., Mabry M., Dykes D. J., Harrison S. D. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cł. Cancer Res. 1992 Sep 1;52(17):4752–4757. [PubMed] [Google Scholar]
- Pan S. S., Andrews P. A., Glover C. J., Bachur N. R. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J Biol Chem. 1984 Jan 25;259(2):959–966. [PubMed] [Google Scholar]
- Phillips R. M., Hulbert P. B., Bibby M. C., Sleigh N. R., Double J. A. In vitro activity of the novel indoloquinone EO-9 and the influence of pH on cytotoxicity. Br J Cancer. 1992 Mar;65(3):359–364. doi: 10.1038/bjc.1992.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989 Aug 15;49(16):4435–4440. [PubMed] [Google Scholar]
- Plumb J. A., Workman P. Unusually marked hypoxic sensitization to indoloquinone EO9 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. 1994 Jan 2;56(1):134–139. doi: 10.1002/ijc.2910560124. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Riley R. J., Workman P. DT-diaphorase and cancer chemotherapy. Biochem Pharmacol. 1992 Apr 15;43(8):1657–1669. doi: 10.1016/0006-2952(92)90694-e. [DOI] [PubMed] [Google Scholar]
- Robertson N., Stratford I. J., Houlbrook S., Carmichael J., Adams G. E. The sensitivity of human tumour cells to quinone bioreductive drugs: what role for DT-diaphorase? Biochem Pharmacol. 1992 Aug 4;44(3):409–412. doi: 10.1016/0006-2952(92)90429-m. [DOI] [PubMed] [Google Scholar]
- Siegel D., Gibson N. W., Preusch P. C., Ross D. Metabolism of diaziquone by NAD(P)H:(quinone acceptor) oxidoreductase (DT-diaphorase): role in diaziquone-induced DNA damage and cytotoxicity in human colon carcinoma cells. Cancer Res. 1990 Nov 15;50(22):7293–7300. [PubMed] [Google Scholar]
- Smitskamp-Wilms E., Peters G. J., Pinedo H. M., van Ark-Otte J., Giaccone G. Chemosensitivity to the indoloquinone EO9 is correlated with DT-diaphorase activity and its gene expression. Biochem Pharmacol. 1994 Apr 20;47(8):1325–1332. doi: 10.1016/0006-2952(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Stephens M. A. The differential hypoxic cytotoxicity of bioreductive agents determined in vitro by the MTT assay. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):973–976. doi: 10.1016/0360-3016(89)90898-5. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Smith P. J., Workman P. The role of NAD(P)H: quinone reductase (EC 1.6.99.2, DT-diaphorase) in the reductive bioactivation of the novel indoloquinone antitumor agent EO9. Cancer Commun. 1991 Jul;3(7):199–206. doi: 10.3727/095535491820873164. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Workman P. Enzymology of the reductive bioactivation of SR 4233. A novel benzotriazine di-N-oxide hypoxic cell cytotoxin. Biochem Pharmacol. 1990 Jun 1;39(11):1735–1742. doi: 10.1016/0006-2952(90)90119-6. [DOI] [PubMed] [Google Scholar]
- Workman P. Bioreductive mechanisms. Int J Radiat Oncol Biol Phys. 1992;22(4):631–637. doi: 10.1016/0360-3016(92)90493-2. [DOI] [PubMed] [Google Scholar]
- Workman P., Stratford I. J. The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev. 1993 Jun;12(2):73–82. doi: 10.1007/BF00689802. [DOI] [PubMed] [Google Scholar]
- Workman P., Walton M. I., Powis G., Schlager J. J. DT-diaphorase: questionable role in mitomycin C resistance, but a target for novel bioreductive drugs? Br J Cancer. 1989 Nov;60(5):800–803. doi: 10.1038/bjc.1989.364. [DOI] [PMC free article] [PubMed] [Google Scholar]


