Abstract
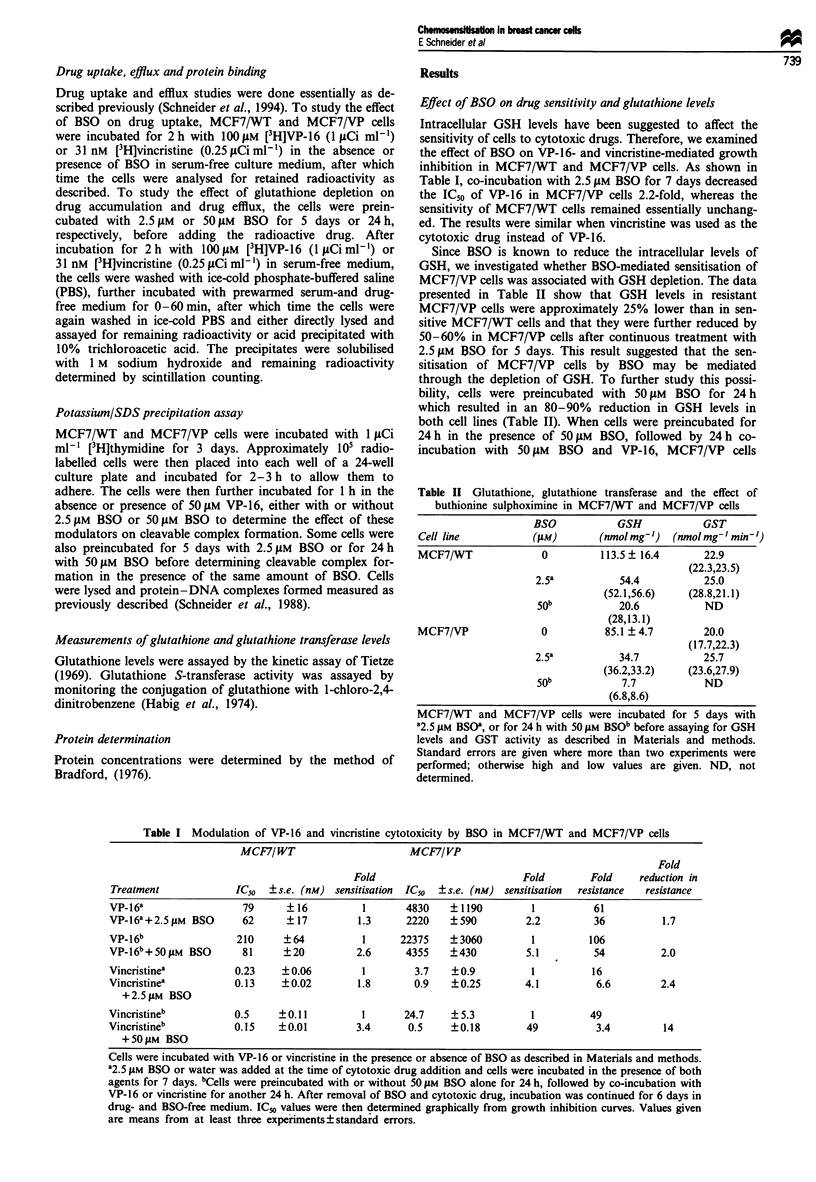
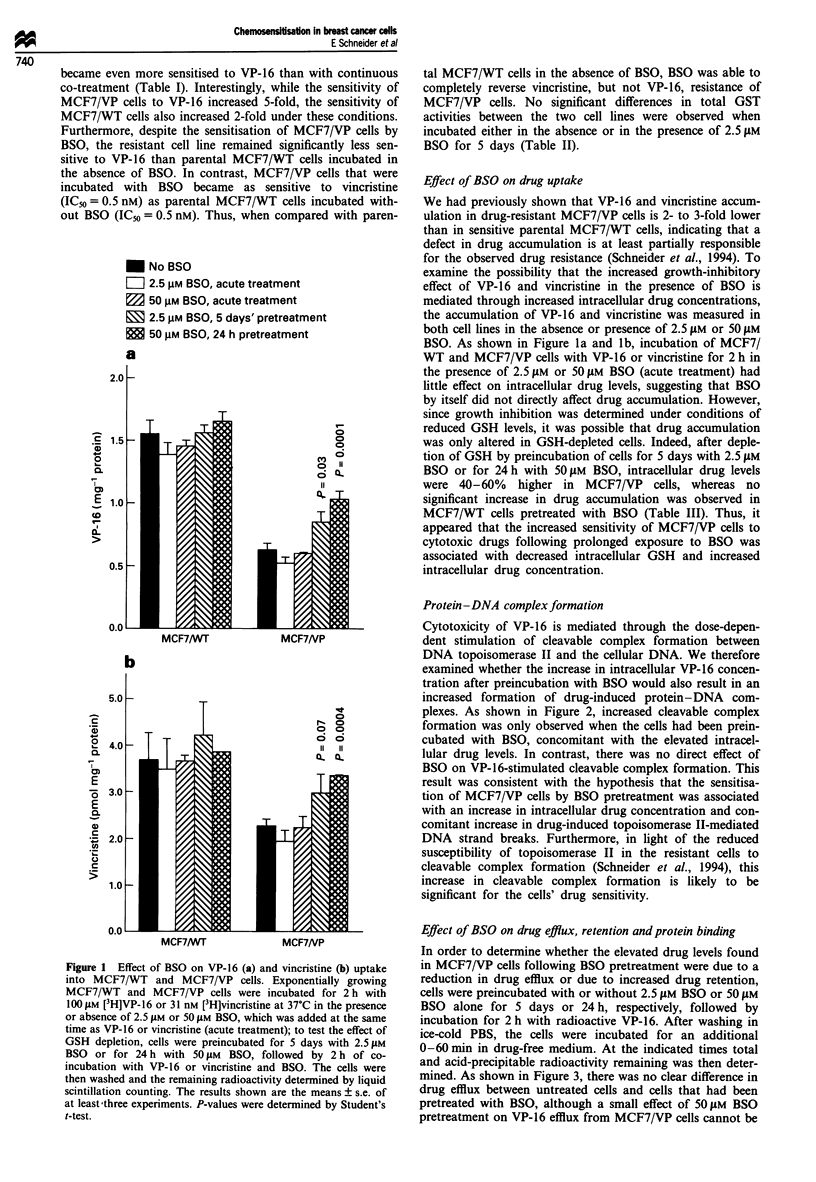
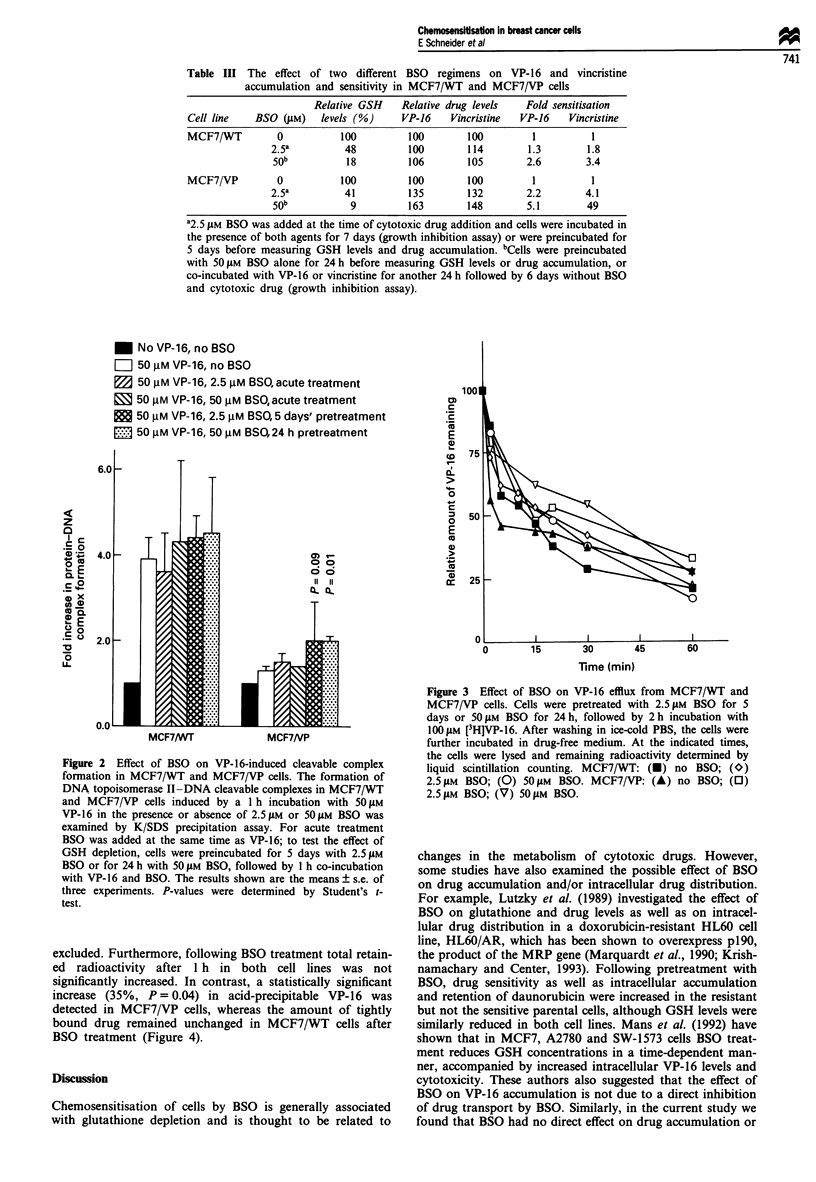
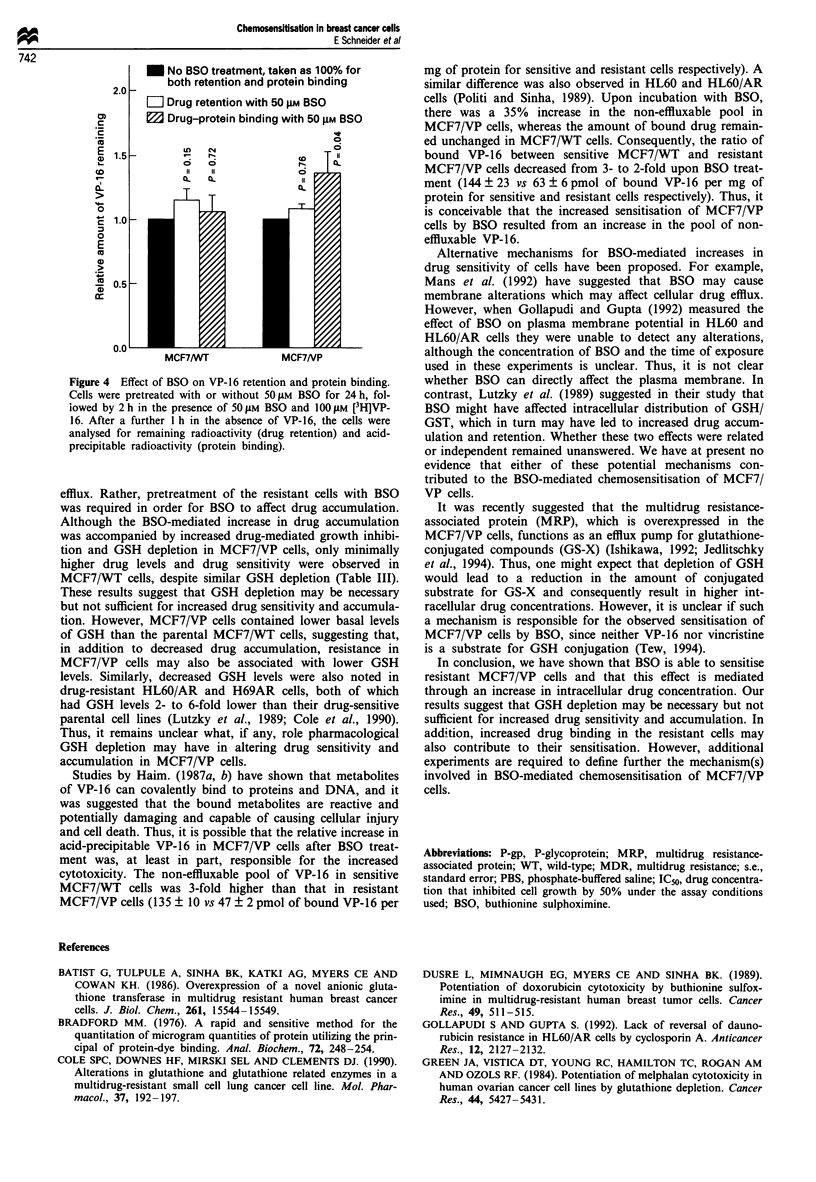
Preincubation of etoposide-resistant human MCF7 breast cancer cells (MCF7/VP) with buthionine sulphoximine (BSO) resulted in their sensitisation to etoposide and vincristine. Chemosensitisation was accompanied by elevated intracellular drug levels. In contrast, simultaneous exposure to BSO did not result in increased drug accumulation. Similar, but quantitatively smaller, effects were also observed when sensitive wild-type MCF7/WT cells were treated with BSO. In agreement with its effect on drug accumulation, BSO pretreatment also increased VP-16-stimulated cleavable complex formation between DNA topoisomerase II and cellular DNA. BSO treatment also led to a significant increase in acid-precipitable VP-16 levels in MCF7/VP, but not MCF7/WT cells. In contrast, no clear effects of BSO on drug efflux were observed and drug retention was only minimally increased after BSO treatment of both MCF7/WT and MCF7/VP cells and no difference between the two cell lines was detected. Thus, chemosensitisation by BSO appeared to be mediated through increased intracellular drug concentrations and/or protein binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Mirski S. E., Clements D. J. Alterations in glutathione and glutathione-related enzymes in a multidrug-resistant small cell lung cancer cell line. Mol Pharmacol. 1990 Feb;37(2):192–197. [PubMed] [Google Scholar]
- Dusre L., Mimnaugh E. G., Myers C. E., Sinha B. K. Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells. Cancer Res. 1989 Feb 1;49(3):511–515. [PubMed] [Google Scholar]
- Gollapudi S., Gupta S. Lack of reversal of daunorubicin resistance in HL60/AR cells by cyclosporin A. Anticancer Res. 1992 Nov-Dec;12(6B):2127–2132. [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Haim N., Nemec J., Roman J., Sinha B. K. In vitro metabolism of etoposide (VP-16-213) by liver microsomes and irreversible binding of reactive intermediates to microsomal proteins. Biochem Pharmacol. 1987 Feb 15;36(4):527–536. doi: 10.1016/0006-2952(87)90362-5. [DOI] [PubMed] [Google Scholar]
- Haim N., Nemec J., Roman J., Sinha B. K. Peroxidase-catalyzed metabolism of etoposide (VP-16-213) and covalent binding of reactive intermediates to cellular macromolecules. Cancer Res. 1987 Nov 15;47(22):5835–5840. [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994 Sep 15;54(18):4833–4836. [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Lutzky J., Astor M. B., Taub R. N., Baker M. A., Bhalla K., Gervasoni J. E., Jr, Rosado M., Stewart V., Krishna S., Hindenburg A. A. Role of glutathione and dependent enzymes in anthracycline-resistant HL60/AR cells. Cancer Res. 1989 Aug 1;49(15):4120–4125. [PubMed] [Google Scholar]
- Mans D. R., Schuurhuis G. J., Treskes M., Lafleur M. V., Retèl J., Pinedo H. M., Lankelma J. Modulation by D,L-buthionine-S,R-sulphoximine of etoposide cytotoxicity on human non-small cell lung, ovarian and breast carcinoma cell lines. Eur J Cancer. 1992;28A(8-9):1447–1452. doi: 10.1016/0959-8049(92)90541-9. [DOI] [PubMed] [Google Scholar]
- Marquardt D., McCrone S., Center M. S. Mechanisms of multidrug resistance in HL60 cells: detection of resistance-associated proteins with antibodies against synthetic peptides that correspond to the deduced sequence of P-glycoprotein. Cancer Res. 1990 Mar 1;50(5):1426–1430. [PubMed] [Google Scholar]
- Moscow J. A., Dixon K. H. Glutathione-related enzymes, glutathione and multidrug resistance. Cytotechnology. 1993;12(1-3):155–170. doi: 10.1007/BF00744663. [DOI] [PubMed] [Google Scholar]
- Politi P. M., Sinha B. K. Role of differential drug uptake, efflux, and binding of etoposide in sensitive and resistant human tumor cell lines: implications for the mechanisms of drug resistance. Mol Pharmacol. 1989 Mar;35(3):271–278. [PubMed] [Google Scholar]
- Schneider E., Darkin S. J., Robbie M. A., Wilson W. R., Ralph R. K. Mechanism of resistance of non-cycling mammalian cells to 4'-[9-acridinylamino]methanesulphon-m-anisidide: role of DNA topoisomerase II in log- and plateau-phase CHO cells. Biochim Biophys Acta. 1988 Mar 31;949(3):264–272. doi: 10.1016/0167-4781(88)90151-0. [DOI] [PubMed] [Google Scholar]
- Schneider E., Horton J. K., Yang C. H., Nakagawa M., Cowan K. H. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994 Jan 1;54(1):152–158. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]



